Key takeaways:
Adbry (tralokinumab-ldrm) and Dupixent (dupilumab) are biologics used to treat moderate-to-severe atopic dermatitis (eczema). Dupixent is FDA-approved for people ages 6 months and older, while Adbry can be used by people ages 12 and older.
Adbry and Dupixent are both injected under the skin. They come in prefilled syringes and injection pens that you can use at home.
Unlike Adbry, Dupixent is approved to treat other health conditions besides eczema. So if you have eczema and one of these conditions, Dupixent may help treat both at the same time.
There are ways to save on Adbry and Dupixent, which are available as brand-name medications. Manufacturer copay cards and patient assistance programs can help make your prescription more affordable.
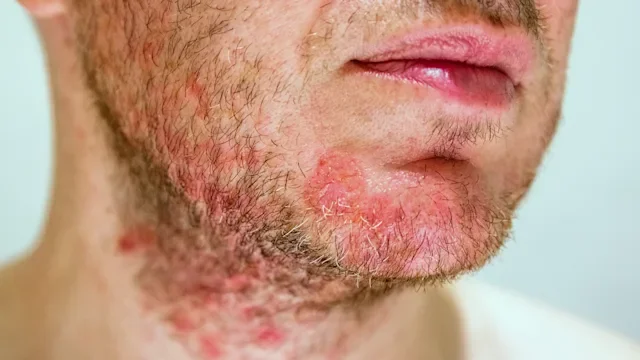
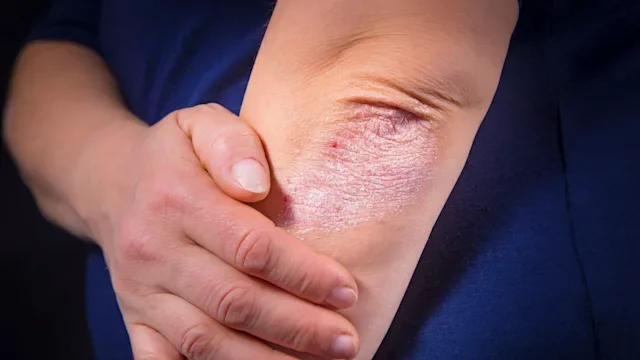
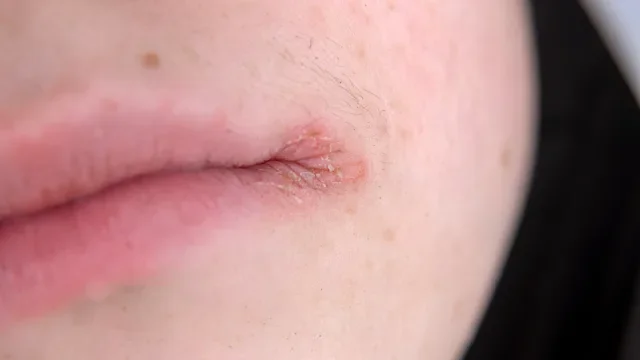
For many people with atopic dermatitis (eczema), topical treatments are often the first line of defense. But when the symptoms become difficult to manage, more targeted treatments may be added on. For those with moderate-to-severe eczema, dermatologists may recommend biologic medications — injectable treatments that can help target the underlying cause of inflammation.
Dupixent (dupilumab) and Adbry (tralokinumab-ldrm) are two biologics for moderate-to-severe eczema. Both treatments can be used alone or with topical treatments, such as corticosteroids. Despite these similarities, Adbry and Dupixent have some key differences. Here are five things to consider.
1. Adbry and Dupixent work slightly differently for eczema
Both Adbry and Dupixent are monoclonal antibodies — lab-made antibodies with specific targets in the body. They work to reduce inflammation by blocking certain proteins in your immune system. Dupixent blocks two proteins, while Adbry blocks one protein.
Both Adbry and Dupixent work by blocking the action of a protein called interleukin-13 (IL-13). IL-13 is found in higher amounts in eczema skin lesions. It plays a key role in creating symptoms such as inflammation and itching.
In addition to IL-13, Dupixent also blocks interleukin-4 (IL-4). IL-4 is also thought to play a role in eczema. And by blocking both IL-13 and IL-4, Dupixent targets multiple steps of the disease process.
2. Dupixent is approved for a wider age range compared with Adbry
Adbry and Dupixent are FDA-approved for different age groups. Both medications are approved for adults. But Adbry is approved for people ages 12 and older, while Dupixent is approved for people ages 6 months and older.
Dupixent was first studied in adolescents and children ages 6 to 11. It was later studied in children ages 6 months to 5 years. All of these studies showed that Dupixent was well-tolerated and helped reduce eczema symptoms.
Adbry’s approval for adolescents is based on a 52-week clinical study that included people ages 12 to 17 with moderate-to-severe eczema. The study showed that Adbry was effective and well-tolerated in this age group. Adbry is currently being studied for eczema in children as young as 6 months, so it could be approved for younger children in the future.
3. Adbry and Dupixent have differences in their dosage schedules
Both Adbry and Dupixent are injected subcutaneously (under the skin), using a prefilled injection pen or prefilled syringe. But there can be some differences in their dosage schedules and how many injections are required for your dose.
Comparing options: If your current eczema treatment isn’t working for your symptoms, here are some eczema creams, pills, and other treatments that your dermatologist may consider for you.
Eczema diet: Learn more about which foods can trigger eczema symptoms — and which ones can help reduce flare-ups.
Real stories: Eczema can show up differently from person to person — with symptoms ranging from mild to severe. Four people describe what it’s like living with eczema.
Adults receiving Dupixent or Adbry usually start with a loading dose (an initial higher dose). The Adbry loading dose uses 4 injections (prefilled syringe) or 2 injections (injection pen). The Dupixent loading dose uses 2 injections. After that, Adbry and Dupixent are injected every other week. But adults with a body weight below 100 kg (220 lbs) who achieve clear or almost clear skin with Adbry after 16 weeks (about 4 months) may be able to inject it every 4 weeks instead.
Dupixent has specific weight-based dosing for children, while Adbry does not. And children under 5 years old receiving Dupixent aren’t recommended to get a loading dose. All other children eligible for Dupixent or Adbry should get a loading dose.
Good to know: After you’ve received training from a healthcare professional, you may be able to give yourself or your child Dupixent or Adbry injections at home. This may be more convenient than going to your dermatologist’s office to get injections.
4. Adbry and Dupixent can cause slightly different side effects
Adbry and Dupixent can cause a few similar side effects, such as injection site reactions (e.g., pain, swelling, and bruising). One study found that people receiving Adbry reported more injection site reactions compared with those receiving Dupixent. But these were generally mild and didn’t stop people from using either medication.
Adbry and Dupixent can also cause eye inflammation. However, Adbry might be less likely to cause eye side effects. Eye problems are common with moderate-to-severe eczema. And treatment with either medication may worsen eye symptoms for some people. But if you’re experiencing eye-related side effects from Dupixent, your dermatologist may consider switching you to Adbry.
Besides injection site reactions and eye inflammation, Adbry and Dupixent can cause a few different side effects in people with eczema. For example, new or worsening joint pain has been reported with Dupixent. And upper respiratory tract infections have been reported with Adbry.
5. Dupixent has approved uses beyond eczema
Adbry is only FDA-approved to treat eczema. But Dupixent has many other approved uses. Other health conditions that Dupixent can be used for include:
Chronic obstructive pulmonary disease (COPD) with high levels of eosinophils (a type of immune cell)
Moderate-to-severe asthma (eosinophilic type or needing oral steroids)
Prurigo nodularis (a skin condition with itchy bumps)
Your dermatologist might consider your other health conditions when prescribing one of these biologic medications for you. For example, some people with eczema may also have asthma. In those cases, Dupixent may be a good option to treat both at the same time.
How to save on Adbry and Dupixent
There are ways to save on Adbry and Dupixent, which are available as brand-name medications. Both medications are typically dispensed through specialty pharmacies, which should help you navigate insurance coverage and savings opportunities.
If you’re eligible, manufacturer copay cards and patient assistance programs (PAPs) can help make your prescription more affordable. With the Dupixent copay card or PAP, you could pay as little as $0 for your prescription. This is also the case with the Adbry copay card and PAP.
The bottom line
Adbry (tralokinumab-ldrm) and Dupixent (dupilumab) are two injectable biologics used to treat moderate-to-severe atopic dermatitis (eczema). Dupixent is approved for other health conditions in addition to eczema. So if you also have one of these other conditions, Dupixent can treat both at the same time.
Both Adbry and Dupixent have some similar side effects, such as eye problems and injection site reactions. But if you’re having side effects from one medication, they might get better if you switch to the other. After reviewing your medical history, your dermatologist can help you decide which option is best for you.

Why trust our experts?



References
Achten, R., et al. (2023). Switching from dupilumab to tralokinumab in atopic dermatitis patients with ocular surface disease: Preliminary case series. Clinical & Experimental Allergy.
Achten, R., et al. (2024). Ocular surface disease in moderate-to-severe atopic dermatitis patients and the effect of biological therapy. Clinical & Experimental Allergy.
American Academy of Allergy, Asthma & Immunology. (2023). Nasal polyps.
Chiricozzi, A., et al. (2020). Targeting IL-4 for the treatment of atopic dermatitis. ImmunoTargets and Therapy.
ClinicalTrials.gov. (2025). A trial to evaluate the efficacy and safety of tralokinumab in combination with topical corticosteroids in children and infants with moderate-to-severe atopic dermatitis (TRAPEDS 2). U.S. National Library of Medicine.
Dupixent. (2024). Frequently asked questions.
Jay, R., et al. (2022). Review of dupilumab-associated inflammatory arthritis: An approach to clinical analysis and management. JAAD Case Reports.
LEO Pharma. (2024). Adbry- tralokinumab-ldrm injection, solution [package insert].
Martora, F., et al. (2024). Injection site reactions after dupilumab or tralokinumab for atopic dermatitis. Journal of Dermatological Treatment.
Napolitano, M., et al. (2023). The hidden sentinel of the skin: An overview on the role of interleukin-13 in atopic dermatitis. Frontiers in Medicine.
Paller, A. S., et al. (2020). Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. Journal of the American Academy of Dermatology.
Paller, A. S., et al. (2022). Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: A randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet.
Paller, A. S., et al. (2023). Efficacy and safety of tralokinumab in adolescents with moderate to severe atopic dermatitis. JAMA Dermatology.
Sanofi-Aventis U.S. (2024). Dupixent- dupilumab injection, solution [package insert]. DailyMed.
Simpson, E. L., et al. (2019). Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis. JAMA Dermatology.