Key takeaways:
Semaglutide is a medication that’s available as an injection (Ozempic, Wegovy pen) and oral pill (Rybelsus, Wegovy pill). It’s FDA approved for Type 2 diabetes, weight loss, and lowering the risk of serious cardiovascular events in certain people. More recently, it has also been approved for uses related to kidney and liver disease.
Researchers have been studying semaglutide for peripheral artery disease and a certain type of heart failure, with potential approvals coming soon.
Ongoing trials are also exploring semaglutide’s potential for polycystic ovary syndrome, substance use disorders, and asthma.
If you're new to using GoodRx for Wegovy savings, pay an introductory price for the first two fills of $199 per month for the injection and $149 per month for the pill (only available for certain doses). Subscribers to GoodRx for Weight Loss can also access FDA-approved, brand-name GLP-1 medications.
Semaglutide (Ozempic, Rybelsus, Wegovy) has quickly become a staple for Type 2 diabetes and weight loss. Known for its effectiveness and versatility, it’s one of the most popular medications in its class. But its story doesn’t stop there.
Ongoing research is uncovering new potential uses, from heart and kidney disease to neurological and metabolic conditions. Here’s what you need to know about semaglutide’s current and future applications.
What is semaglutide?
Semaglutide belongs to the glucagon-like peptide-1 (GLP-1) receptor agonist class. It mimics a natural gut hormone called GLP-1, which helps regulate blood glucose (sugar), appetite, and digestion. It does this by attaching to GLP-1 binding sites (receptors), which are located throughout the body.
Search and compare options
Available as injections (Ozempic, Wegovy pen) and oral pills (Rybelsus, Wegovy pill), semaglutide works by:
Causing the pancreas to release insulin after meals
Reducing glucose production in the liver
Slowing the movement of food out of the stomach
Targeting areas of the brain that regulate appetite and fullness
Researchers are still uncovering other ways semaglutide works in the body, broadening its potential applications.
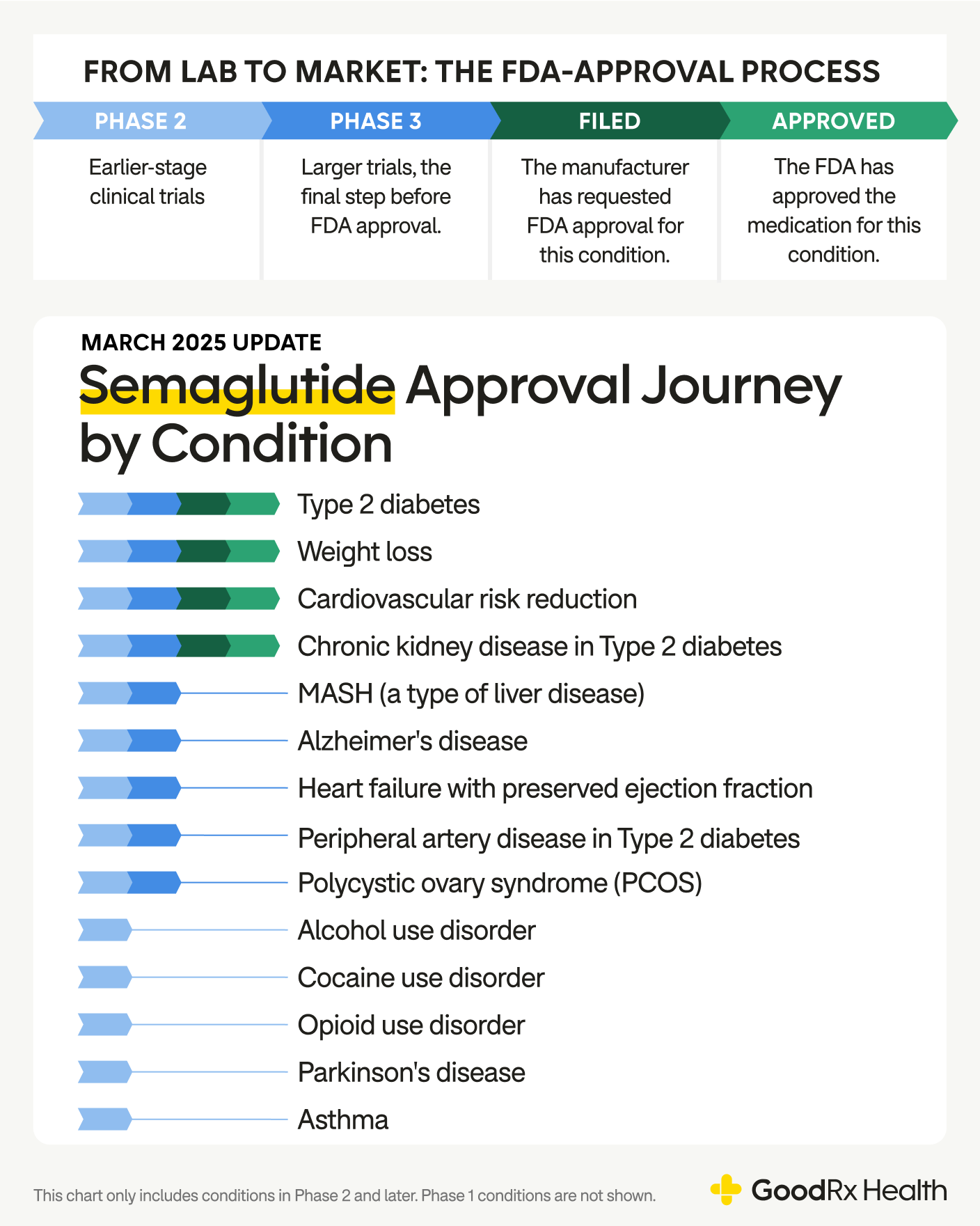
Approved semaglutide uses
Semaglutide is already approved for several key uses. These include Type 2 diabetes, weight loss, and lowering the risk of serious cardiovascular and kidney-related problems in certain people. It's also approved to treat a type of fatty liver disease.
Type 2 diabetes
Semaglutide was first approved as Ozempic in 2017 to manage Type 2 diabetes in adults. The SUSTAIN clinical trials showed its ability to significantly lower hemoglobin A1C (HbA1C or A1C). A1C is a test that measures average blood glucose levels over the last 3 months.
Key SUSTAIN findings include:
A1C reduction ranging from 1.2% to 2.2%, depending on the semaglutide dose
Greater A1C reduction compared to other GLP-1s, including dulaglutide (Trulicity) and extended-release exenatide (Bydureon BCise)
Better blood glucose management as add-on treatment compared to insulin glargine (Lantus) and sitagliptin (Januvia)
Significant reductions in body weight
Compare semaglutide injections: See what experts say about the similarities and differences between Ozempic and Wegovy, two brand-name versions of semaglutide.
How does it work? Learn about how semaglutide works for weight loss.
Possible side effects: Read about semaglutide’s potential side effects — from diarrhea to hair loss — and how to manage them.
Rybelsus, the first oral GLP-1 medication, was approved in 2019. It gave people using Ozempic a needle-free alternative. The PIONEER trials showed that Rybelsus was also effective at lowering A1C and promoting weight loss.
Key PIONEER findings include:
A1C reduction up to 1.4% with the highest dose (14 mg)
Better blood glucose management as add-on treatment compared to sitagliptin and empagliflozin (Jardiance)
Weight-loss benefits, particularly at higher doses
Read more like this
Explore these related articles, suggested for readers like you.
Weight loss
In 2021, semaglutide was approved as Wegovy for weight loss in adults considered obese, or overweight with related health conditions. The following year, Wegovy’s approval was expanded to include certain adolescents age 12 and older.
Results from the STEP clinical trials helped fuel semaglutide’s popularity for weight loss. Key findings showed that semaglutide use was associated with:
An average weight loss of 15% to 17% over 68 weeks (about 16 months)
Greater weight loss compared to liraglutide (Saxenda), another GLP-1 medication for weight loss
Positive effects on health markers such as blood pressure, cholesterol, and blood glucose levels
In late 2025, the FDA approved a pill version of Wegovy for adults. The Wegovy pill, which comes in doses of up to 25 mg, seems to result in weight loss comparable to the 2.4 mg Wegovy injection. If you don't want to give yourself shots, the Wegovy pill offers a needle-free alternative.
Cardiovascular risk reduction
Semaglutide also has proven benefits for people with heart disease. During the SELECT and SUSTAIN-6 trials, semaglutide lowered the risk of major adverse cardiovascular events (heart attack, stroke, or cardiovascular death), or MACE, by 20% and 26%, respectively.
Ozempic is approved to lower the risk of MACE in people with Type 2 diabetes and heart disease. Rybelsus was recently approved for this use in people with Type 2 diabetes at high risk of these events. And Wegovy (injection and pill) is approved to lower the risk of MACE in people with heart disease and a larger body size.
Chronic kidney disease
In January 2025, the FDA approved semaglutide (as Ozempic) to lower the risk of kidney disease worsening, kidney failure, and cardiovascular death in adults with Type 2 diabetes and CKD. Ozempic is the first and only GLP-1 medication approved for this use.
Semaglutide’s kidney benefits have been seen in other studies. But the FLOW trial specifically looked at its impact on major kidney disease events, which included at least one of the following:
Kidney failure
Kidney transplant
Dialysis
Loss of 50% or more of kidney function
Death from kidney-related or cardiovascular causes
Key FLOW findings showed the following with semaglutide compared to placebo (injection without medication):
24% reduction in the risk of major kidney disease events
18% lower risk of MACE
20% lower risk of death from any cause
Slower decline in kidney function
Metabolic dysfunction-associated steatohepatitis (MASH)
In August 2025, the FDA approved Wegovy to treat MASH, a health condition caused by excess fat and inflammation in the liver. MASH is a leading cause of cirrhosis. And until recently, there hadn’t been any FDA-approved treatments.
ESSENCE is a two-part phase 3 trial evaluating semaglutide in adults with MASH and moderate to advanced liver fibrosis (scarring). The first part looked at changes in liver inflammation and scarring, with initial data showing that semaglutide:
Improved liver fibrosis without worsening inflammation (steatohepatitis)
Resolved liver inflammation in many participants
Improved liver function tests
With the support of these findings, Wegovy was granted accelerated approval for MASH. This type of approval allows treatments to become available sooner based on an early marker that suggests a clinical benefit. The second part of the ESSENCE trial will confirm whether these liver improvements lead to fewer serious liver-related events, such as cirrhosis. If they do, the FDA may grant full approval for Wegovy to treat MASH.
Semaglutide pipeline
Semaglutide’s impact extends beyond its current FDA-approved uses. Researchers are exploring its potential to address a variety of other health conditions. Several uses are being studied in phase 3 trials — the last step before the manufacturer applies for approval. Others are in phase 2 trials, which are earlier trials to see if semaglutide works for a specific use.
Here are some of the most promising areas under investigation.
Heart failure with preserved ejection fraction (HFpEF)
Semaglutide is being studied for HFpEF in people considered obese. With HFpEF, the heart muscle contracts normally but is too stiff to fill properly with blood. This can lead to symptoms such as shortness of breath, swelling, and decreased ability to exercise. Having a larger body can worsen symptoms and is thought to be a potential cause of HFpEF.
The STEP-HFpEF and STEP-HFpEF DM phase 3 trials looked at semaglutide’s impact on heart failure symptoms and physical limitations. A pooled analysis of four trials also looked at heart failure events. Here’s how semaglutide stacked up to placebo:
Significantly reduced heart failure symptoms and physical limitations
Improved exercise capacity and 6-minute walk distance
Significant weight loss
Reduced need for diuretics (water pills) to remove fluid buildup
31% lower risk of cardiovascular death or heart failure events
41% lower risk of worsening heart failure events (such as hospitalization)
Semaglutide’s manufacturer resubmitted an application for HFpEF approval in January 2025. An FDA decision could happen in early 2026.
Peripheral artery disease
Peripheral artery disease (PAD) is caused by narrowed blood vessels that reduce blood flow to the limbs. As a common complication in people with Type 2 diabetes, it can increase the risk of serious cardiovascular events, chronic wounds, and amputations. Researchers are now exploring the potential benefits of semaglutide for people with PAD and Type 2 diabetes.
The STRIDE phase 3 trial investigated semaglutide’s impact on walking distance, quality of life, and blood flow to the legs, among other outcomes. At 52 weeks, participants receiving semaglutide showed significant improvements in maximum walking distance, pain-free walking distance, and quality of life compared to those in the placebo group.
The manufacturer has applied for PAD approval, with an FDA decision expected in early 2026.
Alzheimer’s disease
Researchers have been investigating oral semaglutide as a potential treatment for early Alzheimer’s disease. This was prompted by data linking semaglutide to a lower risk of dementia, among other effects. It’s thought that it may have protective and anti-inflammatory effects in the brain.
The EVOKE and EVOKE+ phase 3 trials evaluated whether semaglutide could slow cognitive decline and improve function in early Alzheimer’s disease. Initial results found that it didn't slow progression of the condition. As a result, semaglutide's manufacturer won't be moving forward with the planned 1-year extension of the trial.
Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is a common hormone imbalance in women. It can cause symptoms such as irregular menstrual cycles, weight gain, and infertility. Insulin resistance — when the body doesn’t respond as well to insulin — is a key factor in PCOS.
Early studies have highlighted potential benefits of semaglutide for managing PCOS. This includes improving insulin sensitivity, normalizing menstrual cycles, and causing weight loss. These effects may also improve fertility, which may have contributed to a trend of unexpected pregnancies after starting semaglutide resulting in “Ozempic babies.”
The RESTORE phase 3 trial will help confirm if semaglutide has benefits for women with PCOS. Researchers will look at how it affects ovulation, insulin sensitivity, and other factors. The study includes girls and women ages 12 to 35 with PCOS and a larger body size.
Substance use disorders
You may have heard about people losing interest in drinking alcohol or smoking after starting semaglutide. This surprising effect has sparked research into its potential for treating substance use disorders (SUDs), including alcohol use disorder. Animal studies suggest that GLP-1s may affect the reward system in the brain and reduce cravings.
Several phase 2 trials are looking at semaglutide’s potential role in SUDs, including:
Alcohol use disorder (AUD): Multiple trials are investigating semaglutide’s potential to reduce alcohol consumption and cravings in adults with AUD. These include the STAR and Rybelsus in AUD trials. The SEMALCO trial is focusing on adults with AUD and a larger body size.
Nicotine dependence: The Effects of Semaglutide on Nicotine Intake and Smoking Lapse trial is seeing if semaglutide can reduce cravings and smoking behavior in people with nicotine dependence. Preliminary findings suggest that it’s safe for this use.
Opioid use disorder (OUD): The SHORE trial is looking at semaglutide’s potential in aiding recovering from OUD. The trial focuses on whether it can help manage cravings, improve metabolic health, and support recovery when combined with existing treatments.
Cocaine use disorder: The STAC trial is investigating whether semaglutide can reduce cravings and use in people with cocaine use disorder, including those living with HIV.
Asthma
Having a larger body is a risk factor for developing asthma, a chronic respiratory condition. It can also worsen symptoms and make asthma harder to manage. Emerging research suggests that semaglutide may offer benefits beyond weight loss for certain people with asthma.
The GATA-3 phase 2 trial is investigating semaglutide’s potential in treating asthma. The study focuses on adults with larger body size and asthma that isn’t well-managed with inhaled steroids. Researchers will see if semaglutide can reduce airway inflammation and improve asthma management.
The bottom line
Semaglutide (Ozempic, Rybelsus, Wegovy) is currently FDA approved to treat Type 2 diabetes, support weight loss, treat a type of fatty liver disease, and reduce the risk of serious cardiovascular and kidney-related problems in certain people. But its potential goes far beyond these areas. There has been promising research for heart failure, peripheral artery disease, and a variety of other health conditions. The list of applications is expected to grow as studies uncover new benefits.

Why trust our experts?


References
Ahmann, A. J., et al. (2017). Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with Type 2 diabetes (SUSTAIN 3): A 56-week, open-label, randomized clinical trial. Diabetes Care.
Ahrén, B., et al. (2017). Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. The Lancet Diabetes & Endocrinology.
Ahrén, B., et al. (2018). Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. The Lancet Diabetes & Endocrinology.
American Lung Association. (2016). The link between asthma and weight.
Aroda, V. R., et al. (2017). Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. The Lancet Diabetes & Endocrinology.
Aroda, V. R., et al. (2019). Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1–7 trials. Diabetes & Metabolism.
Aroda, V. R., et al. (2019). PIONEER 1: Randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with Type 2 diabetes. Diabetes Care.
Barnes, J.A., et al. (2020). Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology.
Bergmann, N. C., et al. (2022). Semaglutide for the treatment of overweight and obesity: A review. Diabetes, Obesity & Metabolism.
Carmina, E., et al. (2023). Semaglutide treatment of excessive body weight in obese PCOS patients unresponsive to lifestyle programs. Journal of Clinical Medicine.
Centers for Disease Control and Prevention. (2024). Chronic Kidney Disease.
Cimino, G., et al. (2023). Obesity, heart failure with preserved ejection fraction, and the role of glucagon-like peptide-1 receptor agonists. ECS Heart Failure.
ClinicalTrials.gov. (2018). GLP1R in Parkinson's disease (GIPD).
ClinicalTrials.gov. (2024). A research study investigating semaglutide in people with early Alzheimer's disease (EVOKE).
ClinicalTrials.gov. (2024). A research study investigating semaglutide in people with early Alzheimer's disease (EVOKE Plus).
ClinicalTrials.gov. (2024). Clinical trial of Rybelsus (semaglutide) among adults with Alcohol Use Disorder (AUD).
ClinicalTrials.gov. (2024). Does semaglutide reduce alcohol intake in patients with alcohol use disorder and comorbid obesity? (SEMALCO).
ClinicalTrials.gov. (2024). Effects of semaglutide on nicotine intake.
ClinicalTrials.gov. (2024). Evaluation of semaglutide in adults with cocaine use disorder with and without HIV (STAC).
ClinicalTrials.gov. (2024). Glucagon-like peptide-1 receptor agonist in the treatment of adult, obesity-related, symptomatic asthma (GATA-3) (GATA-3).
ClinicalTrials.gov. (2024). Research study on whether semaglutide works in people with Non-alcoholic Steatohepatitis (NASH) (ESSENCE).
ClinicalTrials.gov. (2024). Role of semaglutide in restoring ovulation in youth and adults with polycystic ovary syndrome (RESTORE).
ClinicalTrials.gov. (2024). Semaglutide for helping opioid recovery (SHORE).
ClinicalTrials.gov. (2025). A research study to compare a medicine called semaglutide against placebo in people with peripheral arterial disease and Type 2 diabetes.
ClinicalTrials.gov. (2025). Semaglutide Therapy for Alcohol Reduction (STAR).
Eren-Yazicioglu. C. Y., et al. (2021). Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Frontiers in Behavioral Neuroscience.
Feldstein, A. E., et al. (2024). Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) overview. American College of Gastroenterology.
Frías, J. P., et al. (2021). Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. The Lancet Diabetes & Endocrinology.
Garvey, W. T., et al. (2024). Efficacy and safety of oral semaglutide 25 mg in adults with overweight/obesity: the OASIS 4 RCT. Novo Nordisk.
Golla, M. S. G., et al. (2024). Heart Failure With Preserved Ejection Fraction (HFpEF). StatPearls.
Kansteiner, F., et al. (2024). Novo rekindles heart failure hopes for semaglutide with new pooled analysis. Fierce Pharma.
Kaplan, A. G., et al. (2022). Asthma exacerbations and glucagon-like peptide-1 receptor agonists: A review of the current evidence. Pulmonary Therapy.
Kolata, G. (2021). ‘A game changer’: Drug brings weight loss in patients with obesity. The New York Times.
Kosiborod, M. N., et al. (2022). Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes, Obesity & Metabolism.
Kosiborod, M. N., et al. (2023). Semaglutide in patients with heart failure with preserved ejection fraction and obesity. The New England Journal of Medicine.
Kosiborod, M. N., et al. (2024). Semaglutide in patients with obesity-related heart failure and Type 2 diabetes. The New England Journal of Medicine.
Kosiborod, M. N., et al. (2024). Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: a pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. The Lancet.
Lincoff, A. M., et al. (2023). Semaglutide and cardiovascular outcomes in obesity without diabetes. The New England Journal of Medicine.
Marso, S. P., et al. (2016). Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. The New England Journal of Medicine.
Mehdi, S. F., et al. (2023). Glucagon-like peptide-1: a multi-faceted anti-inflammatory agent. Frontiers in Immunology.
Novo Nordisk. (2017). Novo Nordisk receives FDA approval of Ozempic (semaglutide) injection for the treatment of adults with Type 2 diabetes. PR Newswire.
Novo Nordisk. (2020). FDA approves Ozempic for cardiovascular risk reduction in adults with type 2 diabetes and known heart disease, updates Rybelsus label. PR Newswire.
Novo Nordisk. (2020). Novo Nordisk to enter phase 3 development in Alzheimer’s disease with oral semaglutide.
Novo Nordisk. (2022). FDA approves once-weekly Wegovy injection for the treatment of obesity in teens aged 12 years and older.
Novo Nordisk. (2024). ESSENCE Phase 3 trial results demonstrating statistically significant and superior improvements with semaglutide 2.4 mg in people with MASH presented at AASLD 2024 - The Liver Meeting.
Novo Nordisk. (2024). Investor presentation Q3 2024.
Novo Nordisk. (2024). Novo Nordisk A/S: Oral semaglutide demonstrates a 14% reduction in risk of major adverse cardiovascular events in adults with type 2 diabetes in the SOUL trial. GlobeNewswire.
Novo Nordisk. (2024). Novo Nordisk A/S: Semaglutide 2.4 mg demonstrates superior improvement in both liver fibrosis and MASH resolution in the ESSENCE trial.
Novo Nordisk. (2024). Ozempic (semaglutide) injection 1 mg demonstrated reduction in risk of kidney disease-related events in Phase 3 FLOW trial presented at the 84th Scientific Sessions of the American Diabetes Association.
Novo Nordisk. (2025). ESSENCE phase 3 trial of semaglutide showed significant improvements at 72 weeks in adults with MASH, published in NEJM. PR Newswire.
Novo Nordisk. (2025). Evoke phase 3 trials did not demonstrate a statistically significant reduction in Alzheimer's disease progression.
Novo Nordisk. (2025). FDA accepts filing application for oral semaglutide 25 mg, which if approved, would be the first oral GLP-1 treatment for obesity. PR Newswire.
Novo Nordisk. (2025). FDA approves Ozempic (semaglutide) as the only GLP-1 RA to reduce the risk of worsening kidney disease and cardiovascular death in adults with type 2 diabetes and chronic kidney disease. PR Newswire.
Novo Nordisk. (2025). Investor presentation Q1 2025.
Novo Nordisk. (2025). Ozempic (semaglutide) injection 1 mg showed improved functional outcomes and health-related quality of life in Phase 3 STRIDE trial in adults with type 2 diabetes and peripheral artery disease (PAD) presented at ACC 2025ery-disease-pad-presented-at-acc-2025-302414859.html. PR Newswire.
O’brien, E. (2024). Efficacy of semaglutide for the treatment of substance use disorders. Psychiatric Times.
Perkovic, V., et al. (2024). Effects of semaglutide on chronic kidney disease in patients with Type 2 diabetes. The New England Journal of Medicine.
Pratley, R. E., et al. (2018). Efficacy of semaglutide vs. dulaglutide across baseline HbA1c in SUSTAIN 7. Diabetes.
Rodbard, H. W., et al. (2019). Oral semaglutide versus empagliflozin in patients with Type 2 diabetes uncontrolled on metformin: The PIONEER 2 trial. Diabetes Care.
Rodbard, H. W., et al. (2020). Efficacy of oral semaglutide: Overview of the PIONEER clinical trial program and implications for managed care. The American Journal of Managed Care.
Rosenstock, J., et al. (2019). Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with Type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: The PIONEER 3 randomized clinical trial. JAMA.
Rubino, D. M., et al. (2022). Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: The STEP 8 randomized clinical trial. JAMA.
Tsoukas, G., et al. (2017). Semaglutide reduces HbA1c across baseline HbA1c subgroups across SUSTAIN 1–5 clinical trials. Canadian Journal of Diabetes.
Tuttle, K. R., et al. (2024). Effects of once-weekly semaglutide on kidney disease outcomes by KDIGO risk category in the SUSTAIN 6 trial. Kidney International Reports.
U.S. Food and Drug Administration. (2019). FDA approves first oral GLP-1 treatment for type 2 diabetes.
U.S. Food and Drug Administration. (2021). FDA approves new drug treatment for chronic weight management, first since 2014.
U.S. Food and Drug Administration. (2024). FDA approves first treatment for patients with liver scarring due to fatty liver disease.
U.S. Food and Drug Administration. (2024). FDA approves first treatment to reduce risk of serious heart problems specifically in adults with obesity or overweight.
Xie, Y., et al. (2025). Mapping the effectiveness and risks of GLP-1 receptor agonists. Nature Medicine.