Key takeaways:
Researchers are studying several weight-loss pills in clinical trials. Medications such as orforglipron and amycretin have notable supporting data so far.
There are other promising new weight-loss drugs in the works too. Injectables such as retatrutide, CagriSema (cagrilintide and semaglutide), and MariTide (maridebart cafraglutide) are in more advanced clinical trials.
More than ten medications are already FDA approved for weight loss. Wegovy (semaglutide) pills and injections, Saxenda (liraglutide), and Zepbound (tirzepatide) are common examples.
There’s not a body out there that’s better than your own. It’s the vehicle that takes you through life. And no matter what size, shape, or height it is, there are countless reasons to be proud of it.
Still, some internal or external factors may be driving you to try to lower your body weight. Perhaps, it’s wanting to improve your body image. Or maybe it’s related to a health issue your prescriber warned you about. Regardless, there are steps you can take to help you meet your health goals.
When possible, a nutritious diet and routine exercise are ideal ways to lose body weight. But, if needed, medication is another way to help promote weight loss. While there are multiple weight-loss medications already on the market, there are also several new weight-loss drugs in clinical trials. Here are 14 prospects to keep in mind.
1. Orforglipron
What it is: Orforglipron is an oral glucagon-like peptide-1 (GLP-1) receptor agonist. It mimics the effects of GLP-1, a gut hormone that’s involved in blood glucose (blood sugar) balance, digestion, and appetite management. Unlike traditional GLP-1 receptor agonists, it’s made of chemicals — not peptides (amino acid chains). This helps it survive in your stomach better.
How it’s administered: Orforglipron is an oral capsule that’s taken once daily.
Status: Orforglipron is in advanced stages of development. It may be approved by March 2026.
Effectiveness: A Phase 3 study shows that adults living with Type 2 diabetes and who are also considered obese or overweight can lose a meaningful amount of weight while taking orforglipron. At the highest dose, people lost about 11% of their body weight on average over 72 weeks (nearly 17 months). This was compared to about 2% with placebo.
Other considerations: Because of its design, some experts predict that orforglipron will be easy to produce in bulk. Meanwhile, competitor GLP-1 receptor agonists, such as Wegovy (semaglutide), are peptides that are relatively hard to make. In turn, this may translate to orforglipron being a more affordable weight-loss medication.
Latest news on orforglipron
December 1, 2025
Convenience and impact: Discover what to know about weight-loss pills that are already FDA approved for use.
Is there a link between gut health and obesity? Your gut microbiome plays a role in your overall health. Learn more about its potential connection to your body weight.
A holistic approach: Combining medication with lifestyle changes can enhance your weight-loss progress. Here, one person shares how a new diet and mindset helped keep him at his ideal weight.
The FDA recently added orforglipron to the second group of medications in its new Commissioner’s National Priority Voucher (CNPV) program. This designation is designed to speed up the review process for certain promising treatments. Eli Lilly, orforglipron’s manufacturer, says it expects U.S. regulatory approval by March 2026.
2. APHD-012
What it is: APHD-012 is an oral medicine that imitates the metabolic effects of gastric bypass (weight loss) surgery. It’s a glucose pill that’s designed to stimulate certain parts of your small intestine.
How it’s administered: APHD-012 is a once-daily oral medication. Its manufacturer is calling it a distal jejunal-release dextrose bead.
Status: APHD-012 is now engaged in its second phase 2 study. It kicked off in September 2025.
Effectiveness: We don’t know exactly how effective APHD-012 will be for weight loss. Stay tuned for updates.
Latest news on APHD-012
October 1, 2024
APHD-012 is also being studied for prediabetes. A phase 2 study found that the medication helps improve glucose tolerance in adults with prediabetes. After taking the medication once daily for 6 weeks, people in the study were able to perform better on an oral glucose tolerance test (OGTT). An OGTT is a diagnostic tool that helps evaluate how well your body is able to process sugars. The medication was also well tolerated overall.
3. ARD-101
What it is: ARD-101 is an oral medication. It targets “bitter taste receptors” in your gut and activates appetite-suppressing hormones such as GLP-1, glucagon-like peptide-2 (GLP-2), and cholecystokinin.
How it’s administered: ARD-101 is an oral pill that’s taken twice daily.
Status: ARD‑101 has completed several phase 2 studies in adults considered obese, people who’ve had weight‑loss surgery, and people with Prader-Willi syndrome (PWS) — a genetic condition that causes a hard-to-satisfy appetite. A phase 3 study to test ARD‑101 for hard-to-treat hunger in PWS is now underway.
Effectiveness: Current data suggests that ARD-101 reduces hunger 2.5 times more than placebo among adults who are considered obese. This may lead to weight loss.
Latest news on ARD-101
March 1, 2025
Aardvark Therapeutics, the company developing ARD-101, became a publicly traded company in February 2025. It plans to use the money raised to continue studying ARD-101 and move it through the steps needed for potential FDA approval.
4. Oral amycretin
What it is: Amycretin is an oral medication that’s being developed for chronic weight management. It’s a dual GLP-1 and amylin receptor agonist. Amylin is a hormone that helps you feel more full after meals.
How it's administered: Amycretin is a once-daily oral tablet. But an under-the-skin amycretin injection is being developed too.
Status: Amycretin is advancing into phase 3 studies. An executive at Novo Nordisk predicted that amycretin will likely be available sometime this decade (before 2030).
Effectiveness: In an early stage study, people taking amycretin lost an average of 13% of their initial body weight after 12 weeks (3 months) of use.
Latest news on amycretin
December 1, 2025
Novo Nordisk shared in June 2025 that it’s moving both the injectable and pill forms of amycretin into phase 3 clinical trials for chronic weight management. The decision came after early studies involving people who are considered obese showed significant and lasting weight loss.
In November 2025, the company announced plans to begin phase 3 studies next year to see how well the medication works for treating diabetes.
5. Monlunabant
What it is: Monlunabant is an oral medication in development for chronic weight management and other metabolic disorders. A small molecule oral cannabinoid receptor 1 (CB1) inverse agonist, it’s thought to help lessen your appetite and support your metabolism.
How it’s administered: Monlunabant is an oral tablet taken once daily.
Status: Monulubant’s manufacturer, Novo Nordisk, released an initial set of phase 2 trial results in September 2024. At this time, the medication’s next steps are somewhat unclear.
Effectiveness: In a phase 2 study, people who took monlunabant lost an average of 15 lbs (7 kg) after 16 weeks (4 months) of use. By comparison, people taking placebo lost a little less than 1 kg (1 lb to 2 lbs).
Latest news on monlunabant
March 1, 2025
As with many other weight-loss medications, monulubant is linked to mild to moderate GI-related side effects. But it may also cause some brain-related side effects, especially at higher doses. Some people taking the medication in clinical trials have reported feelings of irritability, anxiety, and/or sleep troubles. Monlunabant’s side effect profile will continue to be evaluated in upcoming studies.
6. Aleniglipron
What it is: Aleniglipron (GSBR-1290) is another oral GLP-1 medication in development for chronic weight management and Type 2 diabetes. It works similarly to other products in its class.
How it’s administered: Aleniglipron is a once-daily pill.
Status: Aleniglipron’s manufacturer, Structure Therapeutics, released phase 2 study data in late 2025. They shared information from their ACCESS and ACCESS II trials.
Effectiveness: Compared to placebo, aleniglipron helped study participants lose an average of 11% to 15% of their initial body weight after 36 weeks (8 months) of use (depending on the dose).
Latest news on aleniglipron
There’s no other recent news for aleniglipron. Check back with GoodRx for updates.
7. Retatrutide
What it is: Retatrutide is a medication that’s being studied for weight loss and Type 2 diabetes in adults. Retatrutide works by attaching to three receptors (binding sites): GIP (glucose-dependent insulinotropic polypeptide), GLP-1, and glucagon.
How it’s administered: Retatrutide is an injectable medication that’s administered under the skin once weekly.
Status: A phase 3 weight loss study is currently in process. It’s set to finish by early 2026. If data is positive, a FDA approval request will likely follow.
Effectiveness: Eli Lilly, the manufacturer of retatrutide, estimates that the medication may help people lose up to an average of 29% of their initial body weight after about 68 weeks (15 months). It may be even more effective than Zepbound (tirzepatide) for weight loss. Other papers speak to its significant weight loss benefits, too.
Latest news on retatrutide
August 1, 2025
A recently published meta analysis concluded that retatrutide led to the most weight loss among GLP-1-based treatments, but it also caused the most side effects. Dual agonists, such as tirzepatide and survodutide (more details below), produced similar weight loss to retatrutide with fewer side effects. Traditional GLP-1 medications were effective, but resulted in less weight loss overall.
8. CagriSema
What it is: CagriSema is a combination treatment that combines two injectable medications (cagrilintide and semaglutide) into one. Cagrilintide mimics amylin, while semaglutide mimics GLP-1.
How it’s administered: CagriSema is an injectable medication that’s administered under the skin once weekly.
Status: A phase 3 weight loss study started in November 2022. It’s scheduled to run through October 2026.
Effectiveness: CagriSema demonstrated meaningful weight-loss effects in two recent phase 3 studies. After about 68 weeks (15 months), adults without diabetes lost about 20% of their body weight on average. Adults living with Type 2 diabetes saw about 14% weight loss along with improvements in blood sugar readings.
Latest news on CagriSema
July 1, 2025
Novo Nordisk’s phase 3 trials of CagriSema showed strong average weight loss, but the results came in slightly below the company’s earlier goal of about a 25% reduction in body weight. The announcement led to a temporary dip in the company’s stock value as investors adjusted their expectations.
Even so, CagriSema’s results remain notable. Analysts are watching closely to see how it will fit into an increasingly competitive landscape of weight-loss medications.
9. Ecnoglutide
What it is: Ecnoglutide (also called XW003) is another GLP-1 receptor agonist. It’s thought to be a longer-lasting alternative to Saxenda (liraglutide). Saxenda is a once daily, injectable GLP-1 receptor agonist that’s approved for weight loss.
How it’s administered: Ecnoglutide is an injectable medication that’s administered under the skin once weekly. An oral version is also in the works.
Status: Ecnoglutide has been studied in several Chinese phase 3 studies for weight loss and Type 2 diabetes in adults. A new study is now seeing how it stacks up to semaglutide for weight loss.
Effectiveness: In a 40-week (about 9 months) phase 3 study, people using ecnoglutide lost about 9% to 13% of their body weight on average, depending on the dose. Meanwhile, those taking a placebo saw little to no change.
Latest news on ecnoglutide
August 1, 2025
Sciwind Biosciences, the Chinese company developing ecnoglutide, recently stated that its negotiating with an unnamed American company to license the medication in the U.S. The company hopes the partnership will eventually lead to FDA approval and allow the use of existing clinical data from China and Australia to help speed up the approval process.
10. Mazdutide
What it is: Mazdutide (also called IBI362) is a GLP-1 receptor agonist that also mimics glucagon. It’s being studied for weight loss and Type 2 diabetes in adults.
How it’s administered: Mazdutide is an injectable medication that’s administered under the skin once weekly.
Status: Mazdutide has completed several phase 3 studies in China. A new one kicked off in December 2025 and is set to run through 2028.
Effectiveness: In a phase 3 study comparing mazdutide with semaglutide, nearly half of people receiving mazdutide achieved better blood sugar management and lost at least 10% of their body weight within 32 weeks (7 months). With semaglutide, about one-fifth of participants reached those same goals. On average, people lost about 10% of their body weight with mazdutide and about 6% with semaglutide.
Latest news on mazdutide
July 1, 2025
In June 2025, Innovent Biologics announced that China’s regulatory agency approved mazdutide for chronic weight management in adults considered overweight or obese. The approval is based on results from a large phase 3 trial called GLORY-1.
11. Survodutide
What it is: Survodutide activates GLP-1 and glucagon receptors, similar to mazdutide.
How it’s administered: Survodutide is an injectable medication that’s administered under the skin once weekly.
Status: Survodutide completed its phase 2 clinical studies. Phase 3 studies are set to run through April 2026, and the corresponding data should be released during the first half of the year.
Effectiveness: Participants in a phase 2 study lost up to 19% of their initial body weight after using survodutide for about 46 weeks (10 months). Everyone in the study was considered overweight or obese.
Latest news on survodutide
April 12, 2024
Survodutide may be able to treat metabolic dysfunction-associated steatohepatitis (MASH), a form of metabolic dysfunction-associated steatotic liver disease (MASLD). People who are considered overweight or obese have a higher risk for developing MASH.
In a recent 48-week phase 2 study, over 80% of people who received survodutide experienced significant improvements in their liver biopsy reports. By comparison, nearly 20% of people receiving a placebo saw similar results.
12. VK2735
What it is: VK2735 is a GLP-1/GIP receptor agonist medication, similar to tirzepatide.
How it's administered: VK2735 is injected under the skin once weekly. A tablet version of the medication is also in the works, but it’s in earlier stages of development.
Status: Injectable VK2735 has completed its phase 2 trial for weight loss. The study kicked off in September 2023, and it ended in the first portion of 2024. Its 78-week, phase 3 study is actively underway.
Effectiveness: Phase 2 data found that injectable VK2735 helped people lose up to nearly 15% of their initial body weight after 13 weeks (roughly 3 months) of use.
Latest news on VK2735
December 1, 2025
In a phase 2 dosage trial, an oral version of VK2735 helped adults considered obese lose up to about 12% of their body weight in 13 weeks (3 months). People taking a placebo lost only about 1% during the same time. The study met all its main goals, with every dose of VK2735 leading to significantly more weight loss than placebo.
13. MariTide
What it is: MariTide is short for maridebart cafraglutide. It’s an injectable medication that mimics GLP-1 but blocks GIP. This is a different approach from medications such as tirzepatide and VK2735. Some genetic research suggests that blocking GIP in the gut may lead to less fat storage. MariTide is being studied for chronic weight management.
How it's administered: MariTide is injected under the skin. A big part of what makes it unique is its frequency of administration, as it’s only injected once a month.
Status: MariTide is currently enrolled in a phase 2 clinical trial. The study should be wrapped up by early 2026.
Effectiveness: In a phase 2 study, MariTide helped people considered obese lose up to about 20% of their body weight over a year. People living with obesity and Type 2 diabetes saw up to about 17% weight loss; they also saw better blood sugar levels and improvements in metrics such as waist size, blood pressure, and inflammation. Because weight loss was still ongoing at 52 weeks, researchers believe people may lose even more weight with longer treatment.
Latest news on MariTide
October 1, 2024
The CEO of Amgen, the company that’s developing MariTide, noted at a conference that they plan to study MariTide for a variety of weight-related health conditions in phase 3 trials. This includes conditions related to the heart, liver, and kidneys.
Medications that are solely FDA approved for weight loss aren’t typically covered by insurance. Obtaining these additional approvals could potentially pave the way for insurance coverage of MariTide in the future.
14. Eloralintide
What it is: Eloralintide (LY3841136) is a selective amylin receptor agonist in development for weight loss.
How it’s administered: Eloralintide is given as a once‑weekly injection under the skin.
Status: Eloralintide recently completed a phase 2 clinical trial in adults with larger body sizes. Eli Lilly, its manufacturer, plans to begin phase 3 studies by late 2025 to further evaluate its safety and effectiveness for long‑term weight management and related health conditions.
Effectiveness: In a phase 2 study, people receiving weekly eloralintide lost an average of up to 20% of their body weight after 48 weeks (11 months), depending on the dose. The treatment was also linked to improvements in waist size, blood pressure, and cholesterol, all of which play a role in heart health.
Latest news on eloralintide
November 15, 2025
Eli Lilly recently announced that every eloralintide dose group in its phase 2 trial hit the study’s main weight-loss goals. The company is now preparing for phase 3 testing, and many experts are watching to see whether this amylin-based medication could match — or even work alongside — today’s GLP-1 medications for weight loss.
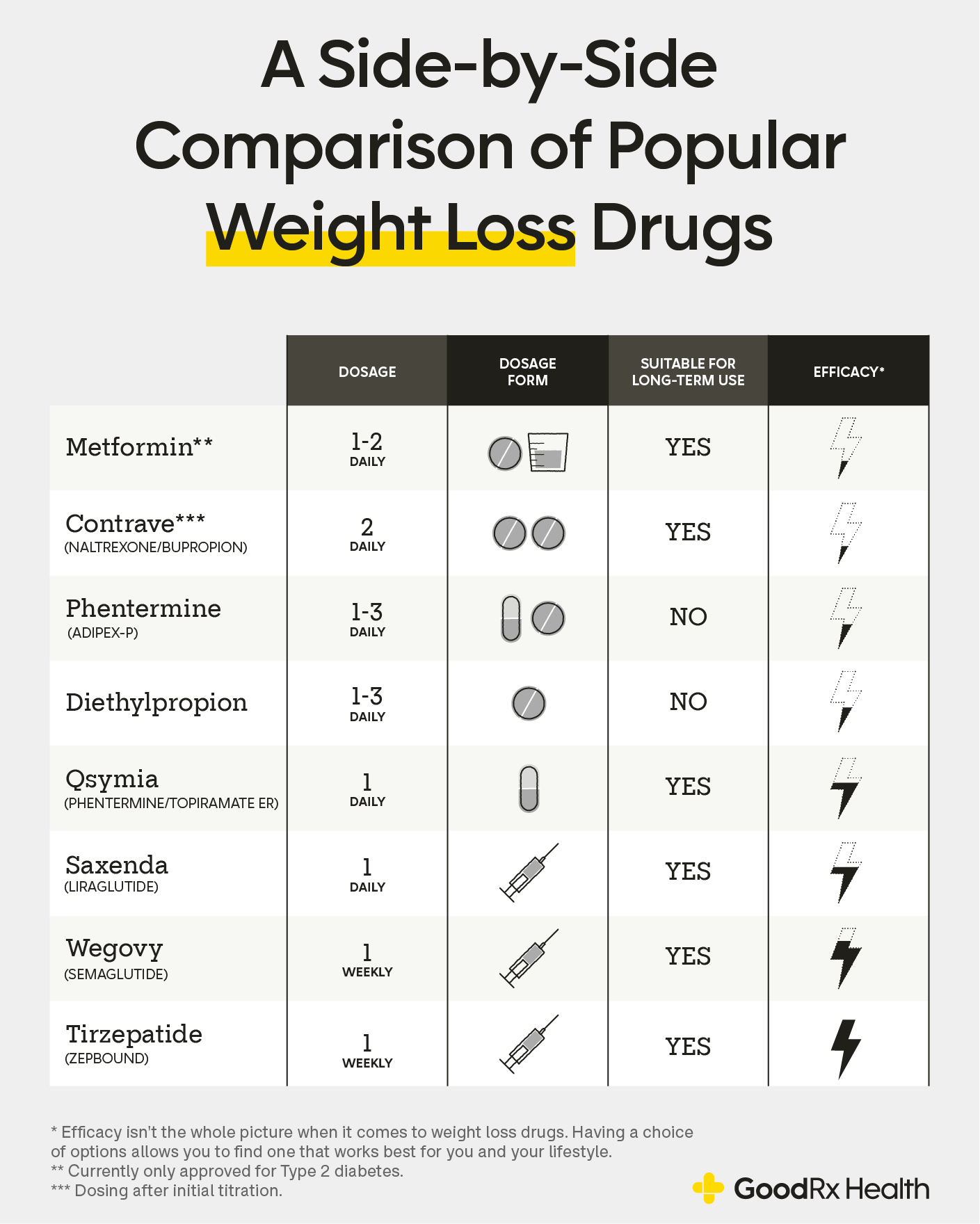
What medications are already FDA approved for weight loss?
As of January 2026, 12 medications are FDA approved as weight-loss treatments. Some are only meant for short-term use, while others can be used for weight management over time.
Medication | Dosage form | How it works |
|---|---|---|
Wegovy (semaglutide) pill | Tablet | Lowers appetite and food intake, helps you feel full |
Contrave (naltrexone / bupropion) | Tablet | Lessens hunger and manages cravings |
Qsymia (phentermine / topiramate ER) | Capsule | Lessens hunger and manages cravings |
Orlistat (Alli, Xenical) | Capsule | Blocks fat absorption from your diet |
Phentermine (Adipex-P, Lomaira)* | Tablet or capsule | Lowers appetite |
Tablet or capsule | Lowers appetite | |
Tablet | Lowers appetite | |
Tablet | Lowers appetite | |
Zepbound (tirzepatide) | Injection | Lowers appetite and food intake, helps you feel full |
Wegovy (semaglutide) injection | Injection | Lowers appetite and food intake, helps you feel full |
Saxenda (liraglutide) | Injection | Lowers appetite and food intake, helps you feel full |
Imcivree (setmelanotide) | Injection | Used only to treat certain genetic conditions, lowers appetite, increases energy use |
*Only meant for short-term use
There are multiple ways to save on FDA-approved weight loss medications, too. For instance, if you're new to using GoodRx for Wegovy savings, pay an introductory price for the first two fills of $199 per month for the injection and $149 per month for the pill (only available for certain doses). For future fills and for other Wegovy doses, pay $349 per month for the injection and $299 per month for the pill.
Are there medications that are used off-label for weight loss?
Yes, many medications are prescribed off label for weight loss. In most cases, they’re prescribed because they cause weight loss as a side effect. These are some top examples:
Trulicity (dulaglutide)
Pramlintide (SymlinPen)
Topiramate (Topamax)
Bupropion (Wellbutrin SR, Wellbutrin XL)
Zonisamide (Zonegran)
Note: You should never take medication for weight-loss purposes without talking to your healthcare professional first. Many of these medications have risks and side effects to keep in mind.
The bottom line
Several new weight-loss drugs are making waves in clinical trials. This includes pills such as orforglipron and amycretin. The same goes for injectable medications such as retatrutide, CagriSema (cagrilintide and semaglutide), and MariTide (maridebart cafraglutide).
Zepbound (tirzepatide), oral and injectable Wegovy (semaglutide), Saxenda (liraglutide), and more are already FDA approved as weight-loss treatments. Your healthcare professional can tell you if weight-loss medication is right for you.

Why trust our experts?


References
Aardvark Therapeutics. (2022). Aardvark Therapeutics announces the initiation of enrollment for three phase 2 clinical trials of oral ARD-101. PR Newswire.
Aardvark Therapeutics. (2023). Aardvark Therapeutics reports positive phase II clinical data for ARD-101. PR Newswire.
Aardvark Therapeutics. (2025). Aardvark Therapeutics announces pricing of initial public offering.
Alvarado, D. (2024). Structure pill leads to competitive weight loss in obesity study. BioPharma Dive.
Amgen. (2025). Results from Amgen's phase 2 obesity study of monthly maritide presented at the American Diabetes Association 85th scientific sessions.
Aphaia Pharma. (n.d.). Our approach.
Aphaia Pharma. (n.d.). Programs.
Aphaia Pharma. (2024). Aphaia Pharma announces positive results from phase 2 trial evaluating its lead drug formulation.
Beaney, A. (2025). Lilly to advance alternative obesity candidate to phase III. Yahoo Finance.
Boehringer Ingelheim. (2023). Data shows nearly 19% weight loss in people with overweight or obesity in Boehringer Ingelheim and Zealand Pharma Phase II trial with survodutide (BI 456906). Business Wire.
Campbell, P. (2025). ADA 2025: CagriSema demonstrates dual benefit in obesity and type 2 diabetes. HCPLive.
Campbell, P. (2025). Slimmer: Ecnoglutide demonstrates weight loss potential in phase 3 trial. HCPLive.
Clinical Trials Arena. (2025). Aphaia doses first subject in second phase II obesity treatment trial.
Clinicaltrials.gov. (n.d.). Search results: Ecnoglutide. National Library of Medicine.
Clinicaltrials.gov. (n.d.). Search results: IBI362. National Library of Medicine.
Clinicaltrials.gov. (2024). A study of IBI362 in participants with obesity or overweight. National Library of Medicine.
Clinicaltrials.gov. (2024). A study to evaluate efficacy and safety of distal jejunal-release dextrose beads formulation (APHD-012) in subjects with a pathological oral glucose tolerance test (OGTT).
Clinicaltrials.gov. (2025). A phase Ib/IIa study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of oral ecnoglutide tablets in Chinese participants with overweight or obesity. National Library of Medicine.
Clinicaltrials.gov. (2025). A research study to see how well CagriSema helps people with excess body weight lose weight (REDEFINE 1). National Library of Medicine.
Clinicaltrials.gov. (2025). A study of IBI362 in Chinese adolescents with obesity or overweight. National Library of Medicine.
Clinicaltrials.gov. (2025). A study of retatrutide (LY3437943) in participants with obesity and cardiovascular disease (TRIUMPH-3). National Library of Medicine.
Clinicaltrials.gov. (2025). A study to compare XW003 injection and semaglutide injection in Chinese adults with obesity (slimmer-up-switch). National Library of Medicine.
Clinicaltrials.gov. (2025). A study to test whether survodutide (BI 456906) helps people living with overweight or obesity who also have diabetes to lose weight (SYNCHRONIZE-2). National Library of Medicine.
Clinicaltrials.gov. (2025). Dose-ranging study to evaluate the efficacy, safety, and tolerability of AMG 133 in adult subjects with overweight or obesity, with or without type 2 diabetes mellitus. National Library of Medicine.
Clinicaltrials.gov. (2025). Research study to investigate how well semaglutide tablets taken once daily work in people who are overweight or living with obesity (OASIS 1) (OASIS 1). National Library of Medicine.
Clinicaltrials.gov. (2025). VK2735 for weight management phase 2 (VENTURE). National Library of Medicine.
Constantino, A. K., et al. (2024). Amgen wants in on the booming weight loss drug market — and it’s taking a different approach. CNBC.
Dehestani, B., et al. (2021). Amylin as a future obesity treatment. Journal of Obesity and Metabolic Syndrome.
Deusch, K., et al. (2025). Coated glucose microbeads stimulate enteric hormone release and improve glucose tolerance in phase 1 and 2 clinical trials. Diabetes, Obesity and Metabolism.
Dunican, K. C., et al. (2010). The role of pramlintide for weight loss. The Annals of Pharmacotherapy.
Eli Lilly and Company. (2025). Lilly's oral GLP-1, orforglipron, is successful in third Phase 3 trial, triggering global regulatory submissions this year for the treatment of obesity.
Eli Lilly and Company. (2025). Lilly's selective amylin agonist, eloralintide, demonstrated meaningful weight loss and favorable tolerability in a Phase 2 study of adults with obesity or overweight. PR Newswire.
Eli Lilly and Company. (2025). Lilly's triple agonist, retatrutide, delivered weight loss of up to an average of 71.2 lbs along with substantial relief from osteoarthritis pain in first successful Phase 3 trial.
Eli Lilly and Company. (2025). What to know about eloralintide: An investigational amylin receptor agonist injection.
Fatima, M. (2025). Aardvark Therapeutics expands phase 3 HERO trial eligibility for ARD-101 in prader-willi syndrome after FDA protocol alignment. Yahoo Finance.
Fick, M., et al. (2024). Novo Nordisk confident of amycretin obesity drug launch this decade. Reuters.
Global Substance Registration System. (2025). Monlunabant. National Center for Advancing Translational Sciences.
Hendricks, E. J. (2017). Off-label drugs for weight management. Diabetes, Metabolic Syndrome and Obesity.
Innovent Biologics. (2025). Innovent announces Mazdutide, first dual GCG/GLP-1 receptor agonist, received approval from China's NMPA for chronic weight management. PR Newswire.
Innovent Biologics. (2025). Innovent's Mazdutide shows superiority in glycemic control with weight loss over semaglutide in a head-to-head phase 3 clinical trial DREAMS-3. PR Newswire.
Katsi, V., et al. (2025). Retatrutide—A game changer in obesity pharmacotherapy. Biomolecules.
Manalac, T. (2024). Amgen plans phase III program for next-gen obesity drug targeting liver and kidney diseases. BioSpace.
Manalac, T. (2024). Boehringer’s obesity candidate survodutide shows strong potential in MASH. BioSpace.
Martinez, R. (2022). Pancreas hormones. Endocrine Society.
Mishra, M., et al. (2024). Viking Therapeutics' weight-loss tablet shows promise in small study. Yahoo Finance.
Naeem, M., et al. (2024). Unleashing the power of retatrutide: A possible triumph over obesity and overweight: A correspondence. Health Science Reports.
Novo Nordisk. (2024). Novo Nordisk A/S: Monlunabant phase 2a trial in obesity successfully completed.
Novo Nordisk. (2025). Novo Nordisk advances early-stage obesity medication, amycretin, to phase 3 clinical development based on early-phase clinical trial results in people with obesity or excess weight, published in The Lancet. PR Newswire.
Reuters. (2025). Eli Lilly expects US FDA approval for oral obesity drug in March 2026.
Samorodnitsky, D. (2025). Obesity-focused aardvark therapeutics files IPO to advance lead candidate. BioSpace.
Sidik, S. (2023). Beyond Ozempic: Brand-new obesity drugs will be cheaper and more effective. Nature.
Silver, A. (2025). Exclusive-China’s Sciwind is in talks to license weight-loss drug in US, CEO says. The Denver Gazette.
Sinha, B., et al. (2025). Efficacy and safety of GLP-1 receptor agonists, dual agonists, and retatrutide for weight loss in adults with overweight or obesity: A Bayesian NMA. Obesity.
Structure Therapeutics. (2025). Structure Therapeutics reports positive topline data from ACCESS program for its once-daily oral small molecule GLP-1 receptor agonist, aleniglipron.
Studna, A., et al. (2025). Oral GLP-1/GIP dual agonist VK2735 achieves up to 12% weight loss in phase II Venture trial. Applied Clinical Trials.
Taylor, N. P. (2025). Novo flunks kidney disease trial, again linking obesity prospect to neuropsychiatric side effects. Fierce Biotech.
Taylor, N. P. (2025). Novo Nordisk expands pivotal amycretin program after dual agonist shines in diabetes. Fierce Biotech.
Tracy, D. (2025). Novo Nordisk’s CagriSema falls short of 25% weight loss target in patients with obesity or overweight. Pharmaceutical Executive.
U.S. Food and Drug Administration. (2025). FDA awards second batch of national priority vouchers.
Véniant, M. M., et al. (2024). A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nature Metabolism.
Viking Therapeutics. (n.d.). VK2735 (subcutaneous & oral formulations) dual GLP-1/GIP receptor agonist.
Viking Therapeutics. (2024). Viking Therapeutics announces positive top-line results from phase 2 Venture trial of dual GLP-1/GIP receptor agonist VK2735 in patients with obesity.
Viking Therapeutics. (2025). Viking Therapeutics announces completion of enrollment in phase 3 Vanquish-1 trial of VK2735. PR Newswire.
Zealand Pharma. (2025). Zealand Pharma announces financial results for the first nine months of 2025. GlobeNewswire.












