Believe it or not, drug recalls happen nearly every week. In the last month alone, we’ve had recalls due to labelling issues, medication mix-ups, and life-threatening adverse effects. While not all recalls are dangerous, here’s how to find out if your drug is recalled and what you should do to stay safe.
Drug recall classes
Drug recalls occur when the quality or safety of a drug has been compromised. It can be due to the drug itself or its packaging and labeling. Quality and safety standards are defined in laws regulated by the FDA.
Most drug recalls are initiated voluntarily by the manufacturer, but on occasion, drug recalls can be requested or mandated by the FDA. After the initial announcement, the FDA categorizes the recall under one of three classes based on how serious the problem is.
Search and compare options
Class I recalls. Class I recalls are the most serious type. The FDA defines a Class I recall as one that involves “a dangerous or defective product that could cause serious health problems or death.”
Example: In 2016, two lots of hyoscyamine tablets were recalled because they were found to contain tablets of non-uniform strength. Some tablets were superpotent, while others were subpotent.
Class II recalls. Class II recalls are the most common type of recall, and they’re not as serious as Class I recalls. According to the FDA, Class II recalls involve “products that might cause a temporary health problem, or pose only a slight threat of a serious nature.”
Example: Earlier this month, one lot of Children’s Advil was recalled due to overdosing concerns related to a labelling error. The dosage cup was marked in teaspoons and the instructions on the label were described in milliliters.
Class III recalls. Class III recalls are the least serious. The FDA defines Class III recalls as those involving “products that are unlikely to cause any adverse health reaction, but that violate FDA labeling or manufacturing laws.”
Example: In 2017, one lot of glipizide extended-release tablets was recalled because it failed limits for water content during stability testing.
What should I do if I think I have a recalled drug?
If you’ve already started taking your drug, your first instinct when you hear that it’s recalled might be to stop taking it right away—but that’s not always the safest decision. Remember, recalls are usually for minor issues. While many over-the-counter drugs can be discontinued at will, some prescription drugs are critical for your health, and you’ll want to continue taking them until your doctor finds a replacement for you.
Follow these steps to stay on the safe side:
1) Get informed.
Make sure your specific medication has been recalled. Generic valsartan, for example, is made by many companies, and recalls usually involve only certain ones. These sites list recall announcements, along with who the manufacturer and distributor of each product is.
Recalls on medications
Recalls and withdrawals of animal and veterinary products
Recalls on biologic products
Recalls on medical devices
For more information, you can contact the manufacturer or FDA directly.
Contact the manufacturer. Manufacturer contact information is usually listed in the FDA recall announcement or on the manufacturer’s website.
Contact the FDA. You can contact the FDA’s Center for Drug Evaluation and Research (CDER) with any questions or concerns at druginfo@fda.hhs.gov or through the following toll-free numbers: 1-855-543-3784 or 1-310-796-3400.
2) Find your medication’s lot number.
Drug recalls pertain to certain lots of the medication that were made during a given time period. To find out which lot numbers were affected by a recall, read the official recall announcement either on the manufacturer’s website or on the FDA’s website here.
Next, you’ll want see if your drug belongs to any of the recalled lots. Here are some tips for finding lot numbers on medication packaging.
Bottles and vials
Drug bottles will often have the lot number printed next to the expiration date, either by the barcode (1) or underneath the dosing instructions (2).

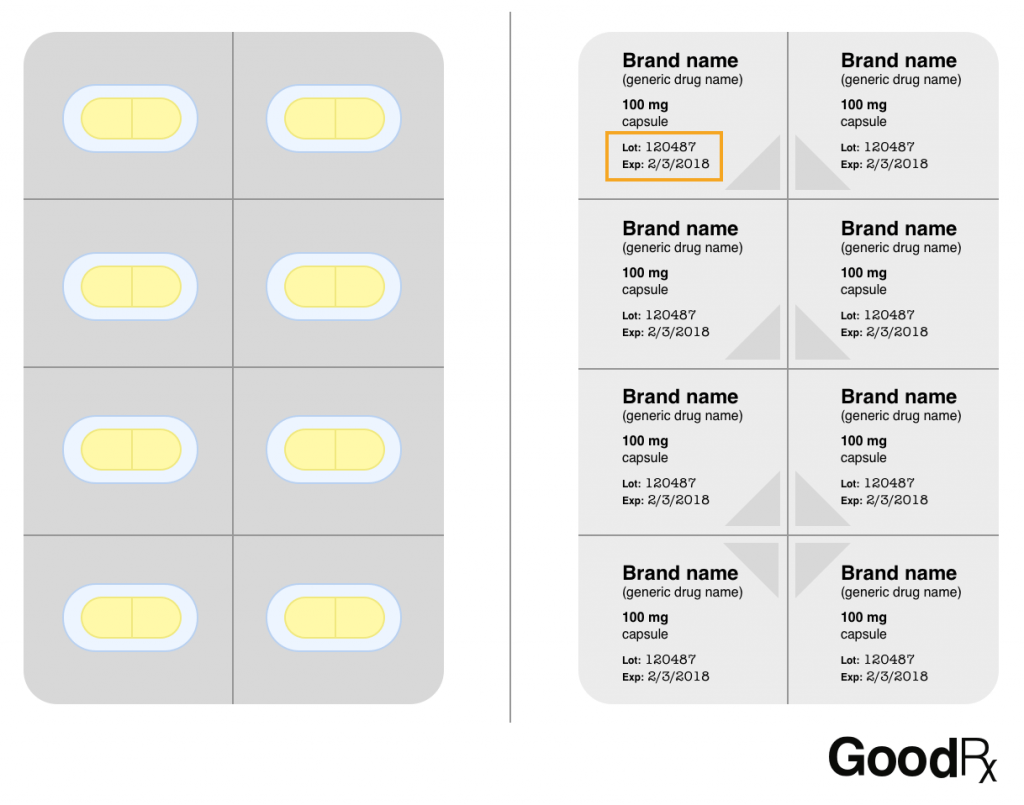
Blister packs
Many blister packs now come with lot numbers and expiration dates printed on their foil backings.
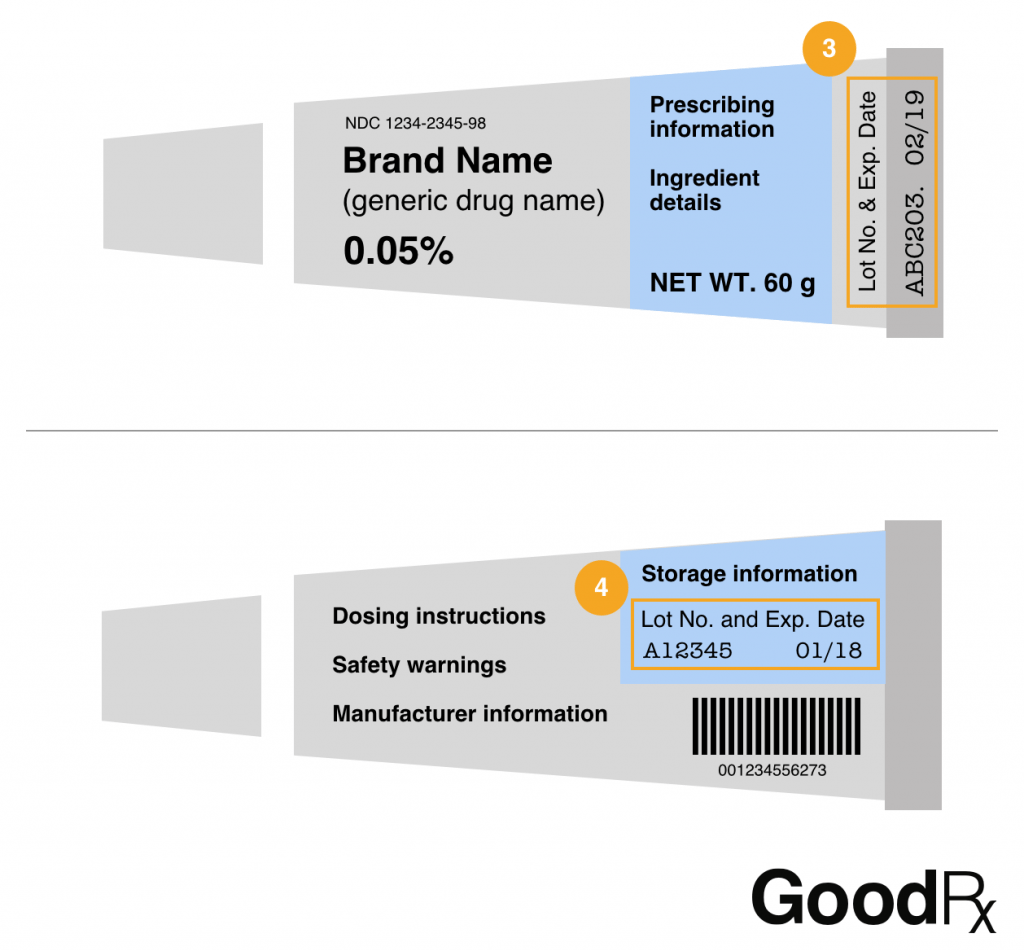
Tubes for creams and gels
Lot numbers on tubes for creams and gels are often printed on or right above the crimp at the end of the tube (3). They can also be on the back of the tube, sometimes hidden in the storage information text (4).
Pharmacy stock containers
Now, if you have a prescription that came in a pharmacy stock container (like a transparent orange pill bottle) without a manufacturer label on it, you’ll need to call your pharmacy to find out your drug’s lot number. Pharmacies keep records of which lots they’ve used and when, so they’ll be able to tell you if you have a recalled medication based on when your prescription was filled.
3) Safely dispose of your medication.
Safely disposing of your recalled medication will reduce the chances of you or others accidentally taking it, and prevent the drug from contaminating the environment. If your medication came with specific disposal instructions, follow those instructions. Some medicines, including controlled substances, need to be taken to an authorized take-back site or flushed down the toilet.
If your medication didn’t come with specific disposal instructions and there are no authorized take-back sites near you, check the FDA’s website on how to dispose of it here. In most cases, you can safely dispose of medications in your household trash. Just remember to mix it in with something you wouldn’t want to eat (like dirt, cat litter, or used coffee grounds) and put that mixture into a sealed plastic bag before tossing it into the trash.
4) Contact your doctor.
Contact your doctor immediately if you experience any unusual symptoms that you think might be related to taking a recalled medication. It’s also a good idea to have your medication packaging and prescription information handy just in case your doctor needs to refer to them.
How are patients notified of recalls?
These days, it’s common to learn about recalls from news outlets and online articles, but there are many other ways in which recall announcements reach the public. Here are some examples:
The FDA, manufacturer or dispensing pharmacy may notify patients by telephone, mail, fax or email if a medication has been recalled.
The manufacturer usually posts recall information on their website.
The FDA may publicly announce a recall via news and other media.
The FDA publishes a weekly Enforcement Report of recalls on their website. You can also subscribe to a newsletter to get this report in your email every week.
The FDA has a Twitter profile, @FDArecalls, where they announce recalls.
– – –
When you bring home any new medication, always inspect it, including all parts of the packaging. If you notice that it doesn’t look or smell right, or if the packaging has been opened or tampered with, contact the manufacturer or your pharmacist for next steps. Any adverse reactions or quality problems can also be reported to the FDA’s MedWatch Reporting Program.

Why trust our experts?















