Key takeaways:
Sophia Kleeh was diagnosed with spinal muscular atrophy before her first birthday after losing her ability to sit up.
Gene therapy provided her with a working copy of a missing gene, halting the progression of the disease.
Now 7, Sophia has surpassed milestones that once seemed impossible.
When Kristina Kleeh’s daughter was 9 months old, she stopped meeting her developmental milestones and could no longer sit up without help.
“She had lost her reflexes, like in her knees,” Kristina says. “She was alert and talkative, but just very floppy and had very low muscle tone.”
Shortly before her first birthday, Sophia was diagnosed with spinal muscular atrophy (SMA), a genetic disorder that causes progressive muscle weakness. She had Type 2, an intermediate form of SMA, which typically prevents children from walking independently.
Kristina remembers the initial shock when she learned of the diagnosis. Doctors said not to search for it on the internet. “But I did,” says Kristina, who lives in Medina, New York, and is the executive director of a nonprofit overseeing childcare programs in schools. “What stood out was that it’s the No. 1 genetic cause of death in children under the age of 2.”
What is spinal muscular atrophy?
Spinal muscular atrophy is caused by mutations in the SMN1 (survival of motor neuron 1) gene, which produces a protein critical for motor neuron health. Without this protein, motor neurons deteriorate, leading to muscle atrophy and weakness.
When Sophia was diagnosed, Kristina and her husband learned that they both carry the genetic mutation that causes spinal muscular atrophy. This means that any child they have would have a 25% chance of inheriting two faulty copies of the gene and developing SMA.
That’s what happened with Sophia. She was born without the SMN1 gene, which produces a vital protein that keeps motor neurons healthy.
“It’s a progressive disease, so it gets worse as you get older,” Kristina says. “It’s kind of like Lou Gehrig’s disease in that you lose your abilities. Ultimately, you lose your ability to breathe and swallow.”
How gene therapy can be life-changing for people with SMA
After Sophia’s diagnosis, Kristina learned there was a drug trial for children with SMA at Nationwide Children’s Hospital in Columbus, Ohio. Within 30 days of diagnosis, Sophia was accepted into the trial. She received the gene therapy medication Zolgensma (onasemnogene abeparvovec-xioi).
What is gene therapy, and how does it change lives? Read how gene therapy can offer cutting-edge treatment options for conditions such as blood cancer.
How can gene therapy turn around a cancer treatment? For this woman, it was the answer for treating acute lymphocytic leukemia.
What should you know about spinal muscular atrophy? Thanks to advancements in gene therapy, new treatment options can slow the progression of muscle weakness.
The one-time treatment uses a virus (AAV9, adeno-associated virus type 9) as a delivery vehicle to transport a working copy of the missing SMN1 gene into the body’s cells. This allows the cells to produce the essential protein, halting SMA progression. Sophia participated in phase 3 of the trial in September 2018.
For Kristina and her husband, the decision to enroll their daughter into the trial was clear.
“It was a pretty easy decision because there was no alternative,” Kristina says. “The alternative was so horrible, so that made it easy.”
Read more like this
Explore these related articles, suggested for readers like you.

They did research, consulted neurologists, talked with families who had gone through something similar, and weighed their options.
“We decided that this was really the absolute best option — to throw a Hail Mary — for her survival and to hope for some quality of life,” Kristina says.
A new lease on life thanks to advancements in gene therapy
Kristina says gene therapy was transformative for Sophia, helping her reach milestones they never thought possible.
“I’m so grateful,” Kristina says. “It’s been all these years, and I still get a little choked up. I’m just so grateful for the scientists and the doctors and nurses and everyone that dedicated decades of their lives toward coming up with this treatment, and especially the other families that had to make the choice before we did to determine whether it was even safe in the first place.”

When Sophia was diagnosed, SMA was the leading genetic cause of death in children under age 2. But the approval of gene therapies like Zolgensma has significantly improved outcomes. Survival rates and motor function for children with SMA are better with this improved treatment.
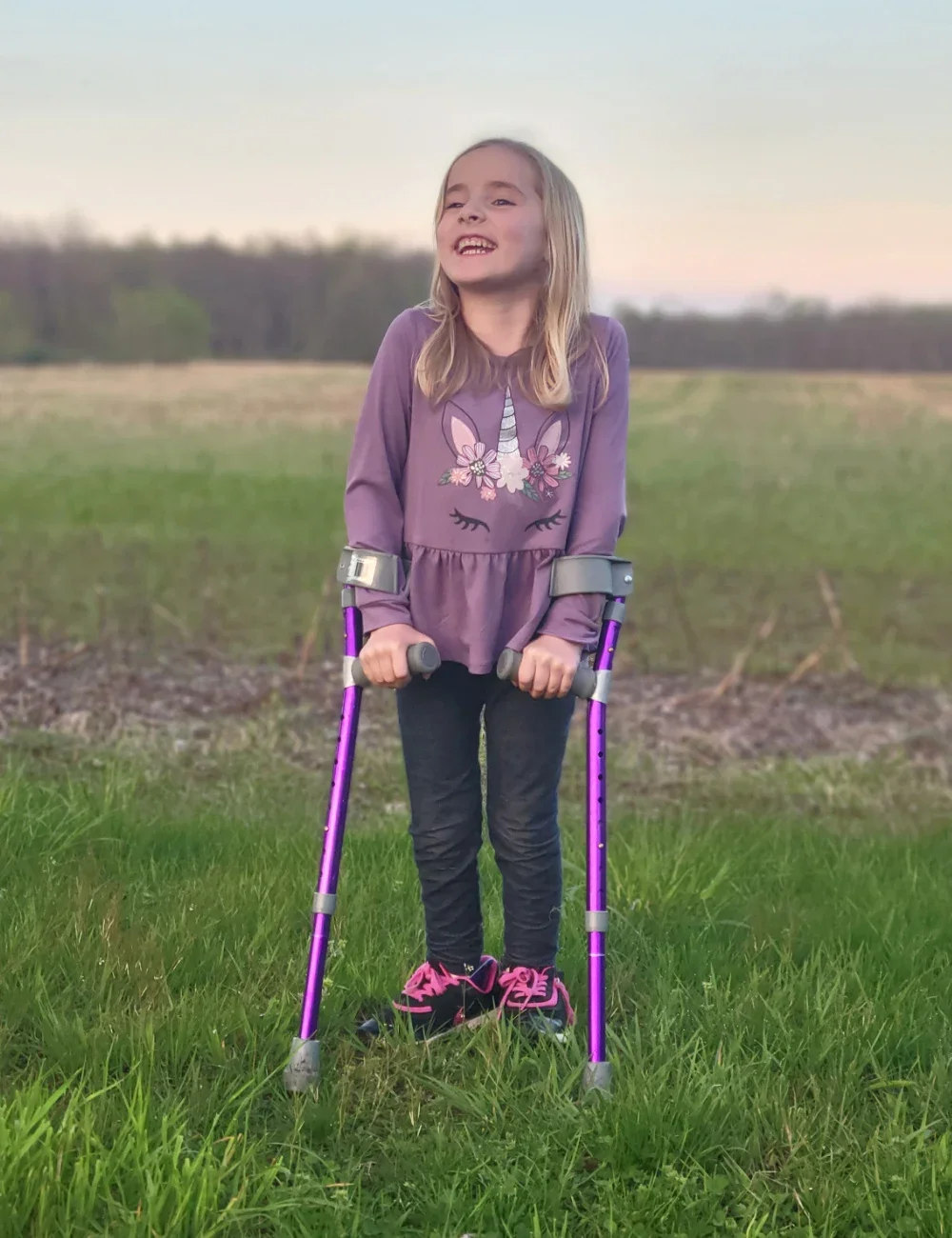
Now 7 years old, Sophia uses a walker or forearm crutches to get around.
“She is delightful. She is bright. She is sweet, and so verbal. She has tons of friends,” Kristina says. “She’s a good, sweet little second grader. She’s really a joy.”
Without treatment, Kristina says Sophia’s life would have been vastly different. She likely wouldn’t have walked.
“She might never have been able to crawl,” Kristina says. “If she was able to sit on her own without support, that would have probably been about where she topped out. And hopefully, you retain the ability to use your arms to feed yourself and that sort of thing. But she absolutely would have been in a motorized wheelchair.”
Today, Sophia enjoys adaptive horseback riding, known as hippotherapy, which helps strengthen muscles and improve balance.
“The walking motion of the horse is as close to actually walking as possible,” Kristina says. “It works similar muscles and balance.”
And riding their horse, Ruby Rose, has given Sophia more freedom than anything else, Kristina says.
Why newborn screening for SMA matters
Since Sophia’s birth, newborn screening for SMA has become standard in the U.S.
“Had she been diagnosed at birth, she would have received treatment by the time she was about 1 month old,” Kristina says. “And if you receive the treatment before the progression of the disease, you can halt it in its tracks.”
Gene therapy’s growing field of hope


For families like Kristina’s, expanding gene therapies means there is more hope.
SMA is one of the many conditions benefiting from gene therapy. The FDA has approved 36 gene therapies, with hundreds more in development for conditions such as:
When the risks of gene therapy are lower than the risks from disease progression, that makes gene therapy a clear choice, Kristina says.
“If you speak with your trusted professionals, the neurologists and researchers, and if the prognosis is more grim than the potential risk, then I think you don’t really have much of a decision to make. It’s a no-brainer.”

Why trust our experts?



















