Is a Herpes Vaccine Currently In the Works?
Key takeaways:
Potential vaccines for herpes simplex virus (HSV) may prevent or treat symptoms of an HSV infection.
There are a few HSV vaccines currently being studied in humans. Examples include treatment vaccines from Moderna, and a preventative vaccine from BioNTech.
HSV vaccines are being tested, but so far none have been approved for use in humans.
Herpes simplex viruses (HSV) are part of a larger family of herpesviruses. They’re very common — affecting about 90% of adults worldwide. And they can cause painful sores in or around the mouth or genitals. Unfortunately, there’s no cure for HSV infections, and people need to manage their outbreaks with medications. Several HSV vaccines have been tested. But none have been brought to market at this point.
Yet companies are still trying to figure out how to prevent these infections through vaccination — and with good reason. Modeling suggests that an HSV vaccine for the virus that commonly causes genital herpes could prevent as many as 350,000 new infections every year.
There are several HSV vaccine candidates in the pipeline. The hope is that they’ll show promise where previous prospects have fallen short. Here, we’ll discuss new and ongoing research in this area and if we can expect a vaccine to be available in the near future.
What is the herpes simplex virus (HSV)?
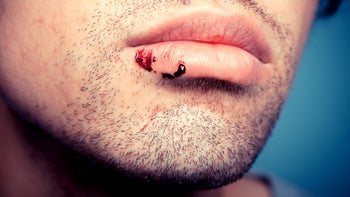
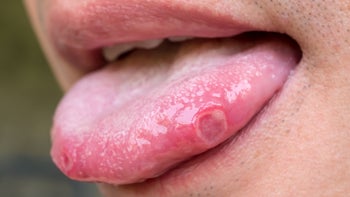
HSV is a virus that can cause painful ulcers in your mouth (cold sores) or on your genitals. There are two main herpes simplex viruses — HSV-1 and HSV-2. HSV-1 infections most commonly affect your mouth, but they may also result in sores on your genitals. HSV-2, on the other hand, primarily affects your genitals.
Once you’ve been infected with HSV, the virus stays in your body long term. This can cause sores or blisters to appear periodically (called an outbreak). In rare cases, it can cause life-threatening infections like meningitis and encephalitis.
When you think of herpesviruses, HSV is likely what comes to mind. But both types of HSV are actually part of a larger family of herpesviruses, including:
Varicella-zoster virus (causes chickenpox and shingles)
Epstein-Barr virus (causes mononucleosis)
Search and compare options
Is there a cure for herpes?
Unfortunately, there’s no cure for herpes at this time. However, researchers are hoping that gene therapy could potentially open the door for a future herpes cure. So far, results from animal studies have been promising.
Until then, HSV infections are managed with prescription antiviral medications, like Valtrex (valacyclovir) or Zovirax (acyclovir). These medications can help resolve blisters when they appear. And some people take them daily to prevent outbreaks, too.
However, some people may find that available medications don’t work well for them. And condoms may not cover all areas where genital blisters appear to avoid transmitting the infection to your partner. This has prompted research into whether a vaccine for these viruses is possible.
Why is creating an HSV vaccine so difficult?
Herpesviruses can live in your body undetected. This means your immune system doesn’t react to them right away. The longer the virus goes undetected, the more difficult it is to make an effective vaccine. Even though we have vaccines for other illnesses caused by herpesviruses, such as chickenpox and shingles, HSV does a better job of evading the immune system.
When scientists make vaccines, they target the part of your immune system that recognizes an invader — like a virus or bacteria — in your body. The vaccine then teaches your body how to kill the invader before it makes you sick. If the immune system doesn’t react to HSV right away, a vaccine cannot teach your body how to protect you.
HSV vaccine studies in animals have had good results. But when these vaccines were studied in humans, they didn’t work as well. That’s when pharmaceutical companies had to restart their research attempts.
What do you need to know if you have genital herpes? It doesn’t have to be a life-changing diagnosis.
How do you overcome the stigma of herpes? This North Carolina man started an organization to help others.
What are the chances of getting herpes from an infected partner? The short answer is: It depends on a number of factors.
Has an HSV vaccine ever been tested on humans?
Yes. Human studies of several vaccines have occurred in the last few years. People who participated in the vaccine trials didn’t have many side effects. But they didn’t see much benefit either. Even though earlier studies didn’t work out, several vaccines are still being tested.
Are there any herpes (HSV) vaccines currently being studied?
Yes, vaccines are currently being researched to target both HSV-1 and HSV-2. Most are being developed for HSV-2. But since the viruses are so similar, an HSV-2 vaccine would also likely work to prevent or treat HSV-1, too.
There are two types of HSV vaccines being studied:
Treatment vaccines: These vaccines would benefit people who’ve already been infected with HSV. They could improve symptoms, help blisters heal faster, and lower the risk of spreading the virus to other people.
Preventative vaccines: These vaccines would benefit people who haven’t been exposed to HSV. They would train your immune system to prevent an HSV infection if you were exposed to the virus in the future.
Just as with any vaccine, all HSV vaccines will need to go through three types of clinical trials — phase 1, 2, and 3 trials — before being approved by the FDA. This to make sure that they’re safe and effective for humans. This process typically takes several years. So, it will likely be some time before we see an HSV vaccine come to market. Here are a few HSV vaccine candidates being studied right now.
mRNA-1608
Messenger RNA (mRNA) technology has shown potential for vaccines against several types of infections — including HSV. Moderna is working on an mRNA HSV-2 treatment vaccine known as “mRNA-1608.”
Currently, the mRNA-1608 vaccine is being studied in a phase 1/2 clinical trial in adults ages 18 to 55 with a history of recurrent HSV-2 genital herpes. Researchers are testing several different doses to assess safety and immune response. The study is expected to be completed in June 2025.
BNT163
BioNTech’s HSV-2 vaccine (BNT163) is also making progress. It uses mRNA technology to prevent infection. The company launched a phase 1 clinical trial in December 2022 studying the vaccine in adults ages 18 to 55 without a history of symptomatic genital herpes. The study is expected to be completed in December 2025.
RVx-201
RVx-201 is a treatment vaccine developed by Rational Vaccines. In animal studies, it was shown to significantly reduce the number of symptomatic days by 45% compared to 24% with a different HSV vaccine. It also reduced recurrent genital herpes lesions.
In 2022, the company launched a vaccine candidate study. The study was intended to help researchers determine baseline characteristics of participants and their willingness to participate in a clinical trial. The study has since been completed, but no further updates are available. The company is also researching preventative vaccines for HSV-2 (RVx-2001) and HSV-1 (RVx-1001).
The bottom line
Studies are underway to find vaccines for the treatment or prevention of herpes simplex virus (HSV) infections. HSV vaccines would benefit millions of people. To make sure vaccines are safe and effective, they must pass through multiple steps before they are approved.
Even though prior vaccines have not been successful in humans, researchers continue to look for new ways to find something that works. As we learn more about HSV infections and how they work, a vaccine may be possible in the future.
Why trust our experts?



References
Aubert, M., et al. (2024). Gene editing for latent herpes simplex virus infection reduces viral load and shedding in vivo. Nature Communications.
Ayoub, H. H., et al. (2020). Epidemiological impact of novel preventative and therapeutic HSV-2 vaccination in the United States: Mathematical modeling analyses. Vaccines.
BioNTech. (2022). BioNTech starts phase 1 clinical trial for prophylactic herpes simplex virus-2 vaccine candidate BNT163.
Centers for Disease Control and Prevention. (2023). Explaining how vaccines work.
Centers for Disease Control and Prevention. (2024). About chickenpox.
Centers for Disease Control and Prevention. (2024). About cytomegalovirus.
Centers for Disease Control and Prevention. (2024). About Epstein-Barr virus (EBV).
Centers for Disease Control and Prevention. (2024). How vaccines are developed and approved for use.
ClinicalTrials.gov. (2023). A study of the characteristics of patients diagnosed with recurrent symptomatic genital herpetic disease. U.S. National Library of Medicine.
ClinicalTrials.gov. (2024). A clinical trial in healthy volunteers to study the safety, tolerability, and immune responses after vaccination with an investigational vaccine designed to prevent genital herpes lesions. U.S. National Library of Medicine.
ClinicalTrials.gov. (2024). A study of mRNA-1608, a herpes simplex virus -2 (HSV-2) therapeutic candidate vaccine, in healthy adults 18 to 55 years of age with recurrent HSV-2 genital herpes. U.S. National Library of Medicine.
ClinicalTrials.gov. (2024). A study on the reactogenicity, safety, immune response, and efficacy of a targeted immunotherapy against HSV in healthy participants aged 18-40 years or in participants aged 18-60 years with recurrent genital herpes. U.S. National Library of Medicine.
Dropulic, L. K., et al. (2012). The challenge of developing a herpes simplex virus 2 vaccine. Expert Review of Vaccines.
Fred Hutch Cancer Center. (2024). Herpes cure with gene editing makes progress in laboratory studies.
Johnston, C., et al. (2016). Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine.
Kaye, K. M. (2023). Overview of herpesvirus infections. Merck Manual Professional Version.
Keown, A. (2022). Rational’s experiental herpes vaccines shows preclinical promise. BioSpace.
Krishnan, R., et al. (2021). Developments in vaccination for herpes simplex virus. Frontiers in Microbiology.
Lutmer, H. (2022). Herpes vaccine candidate launches early-phase study. Precision Vaccinations.
Moderna. (2024). HSV vaccine (mRNA-1608).
Wald, A., et al. (2007). Persistence in the population: Epidemiology, transmission. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press.
Whitley, R. J. (1996). Herpesviruses. Medical Microbiology, 4th Edition. The University of Texas Medical Branch at Galveston.
World Health Organization. (2023). Herpes simplex virus.