How to Find the COVID-19 Pills Paxlovid and Lagevrio
Key takeaways:
Paxlovid (nirmatrelvir / ritonavir) and Lagevrio (molnupiravir) are two oral antiviral treatments for mild to moderate COVID-19. You need a prescription to get these COVID pills, since they aren’t available over the counter.
You can get Paxlovid or Lagevrio by seeing your primary care provider or an urgent care center. You can also visit a Test to Treat location, where they can examine you and provide an antiviral in one visit. A telehealth Test to Treat option is also available.
You may be able to get Paxlovid directly from your pharmacist. To do this, you’ll need to give your pharmacist a copy of your medical records, results from recent kidney and liver labs, and your medication list. This option isn’t available for Lagevrio.
Access savings on related medications
Table of contents

When the FDA authorized two antiviral pills to treat mild to moderate COVID-19, it brought hope for people at high risk of severe illness from the disease. The names of these COVID pills are Paxlovid (nirmatrelvir / ritonavir) and Lagevrio (molnupiravir). The oral antiviral medications promised something that had been missing from COVID treatments: a user-friendly way of intervening early to potentially change the course of the illness.
Paxlovid and Lagevrio aren’t right for everyone who has COVID. They also aren’t a substitute for COVID vaccines. But certain people have higher risks for developing severe symptoms. These include adults age 50 and older, people with certain health conditions, and people with weakened immune systems. These groups of people benefit most from taking Paxlovid or Lagevrio.
There’s no shortage of these COVID pills. But fear of symptom rebound and not knowing how to get Paxlovid or Lagevrio have negatively affected their use. Because of this, many people who are eligible to take COVID pills aren’t getting prescriptions for them.
SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) is now $0 for almost everyone*
Get SHINGRIX at the pharmacy or in-network doctor’s office today. 98% of privately insured people pay $0 and all Medicare Part D beneficiaries pay $0 at the pharmacy.
Prescribing Information
*Coverage and cost may vary and are subject to change without notice. Reimbursement decisions are made by individual insurance plans.


SHINGRIX is an FDA-approved vaccine for the prevention of shingles (herpes zoster) in adults 50 years and older. SHINGRIX is not used to prevent chickenpox.
• You should not receive SHINGRIX if you are allergic to any of its ingredients or had an allergic reaction to a previous dose of SHINGRIX
• An increased risk of Guillain-Barré syndrome (severe muscle weakness) was observed after vaccination with SHINGRIX
• Fainting can happen after getting injectable vaccines, including SHINGRIX. Precautions should be taken to avoid falling and injury due to fainting
• The most common side effects are pain, redness, and swelling at the injection site, muscle pain, tiredness, headache, shivering, fever, and upset stomach
• SHINGRIX was not studied in pregnant or nursing women. Tell your healthcare provider if you are pregnant, plan to become pregnant, or are breastfeeding
• Vaccination with SHINGRIX may not protect all individuals
• Ask your healthcare provider about the risks and benefits of SHINGRIX. Only a healthcare provider can decide if SHINGRIX is right for you
You are encouraged to report vaccine adverse events to the US Department of Health and Human Services. Visit www.vaers.hhs.gov to file a report, or call 1-800-822-7967.
For US audiences.
Trademarks are property of their respective owners.
©️2024 GSK or licensor.
PMUS-SGXWCNT240015 May 2024
Produced in the USA.
GoodRx Health information and resources are reviewed by our editorial staff with medical and healthcare policy and pricing experience. See our editorial policy for more detail. We also provide access to services offered by GoodRx and our partners when we think these services might be useful to our visitors. We may receive compensation when a user decides to leverage these services, but making them available does not influence the medical content our editorial staff provides.
How can I get Paxlovid or Lagevrio?
Paxlovid and Lagevrio are both prescription-only medications. They’re not available over the counter (OTC). That means you’ll need a prescription from a healthcare professional to purchase them. But there are a few ways you can get a prescription for COVID pills.
Good to know: A positive COVID test is not required to get a prescription for Paxlovid. But it is required to receive Lagevrio.
1. Visit a healthcare professional or an urgent care center
The most obvious way to get Paxlovid or Lagevrio is to visit your primary care provider or an urgent care center. After a healthcare professional examines you, they can send a prescription to your pharmacy.
2. Visit a Test to Treat location
Another option for getting Paxlovid or Lagevrio is through the Test to Treat program. The U.S. Department of Health and Human Services started this program in March 2022 to help people access these COVID pills faster.
Participating pharmacy-based clinics, community health centers, and long-term-care facilities can test you for COVID. Then they can provide you a course of Paxlovid or Lagevrio if it’s appropriate for you. There’s also a telehealth Test to Treat option that you can access using a computer or mobile device.
3. Get Paxlovid directly from your pharmacist
You may also be able to get Paxlovid directly from your pharmacist. This option is not available for Lagevrio. Only licensed practitioners, such as a physician, can prescribe Lagevrio.
In July 2022, the FDA authorized pharmacists to prescribe the medication directly to people with mild to moderate COVID. Keep in mind that not all community pharmacies offer this. It’s a good idea to call ahead and ask if your pharmacy provides this service.
If your pharmacist will be prescribing Paxlovid for you, you’ll need to bring some documents with you:
A digital or paper copy of your medical records (must be less than a year old)
A digital or paper copy of your most recent liver and kidney function tests (must be less than a year old)
An up-to-date medication list that includes all prescription and OTC medications, vitamins, and supplements that you take
Paxlovid versus Lagevrio: Pharmacists highlight the differences between the two COVID-19 pills.
What it feels like: Real people share their experiences with Paxlovid, which can be beneficial for many people at high risk from COVID.
Other COVID treatments: Read about what other options are available for treating and preventing COVID.
If you don’t have this information readily available, your pharmacist may be able to contact your primary care provider to obtain it. But this can cause delays in how soon you can start the medication. In this case, you may be better off following one of the first two tips listed above.
What is Paxlovid?
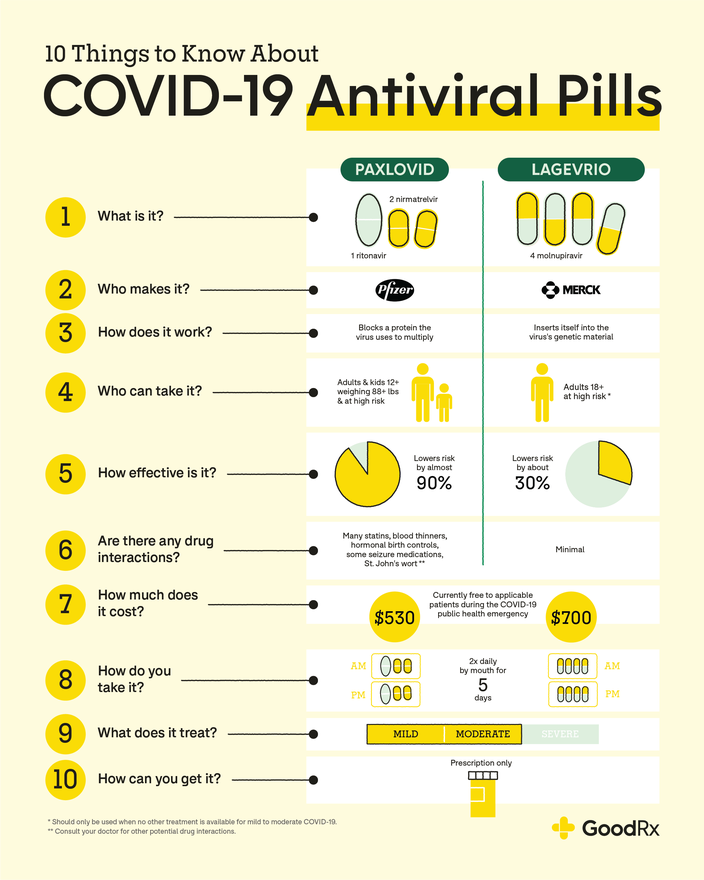
Paxlovid is a combination of two antiviral medications: nirmatrelvir and ritonavir. Nirmatrelvir helps stop SARS‑CoV‑2 (the coronavirus that causes COVID) from multiplying in your body. Ritonavir helps boost nirmatrelvir’s actions so that it sticks around in the body for longer and at higher levels.
Paxlovid is FDA approved for adults with mild to moderate COVID who are at risk of developing severe symptoms. It’s also FDA authorized to treat mild to moderate COVID in children age 12 and older who weigh at least 88 lbs and are at risk for developing severe illness.
The typical Paxlovid dosage for most people is 3 pills (2 nirmatrelvir pills and 1 ritonavir pill) by mouth twice a day for 5 days. People with kidney problems may need to take a lower dosage. And people with severe liver disease shouldn’t take it. You should start taking Paxlovid within the first 5 days of feeling COVID symptoms.
Test your knowledge about Paxlovid
What is Lagevrio (molnupiravir)?
Lagevrio is an oral antiviral pill that contains the medication molnupiravir. It also stops SARS‑CoV‑2 from multiplying, but in a different way from Paxlovid. Lagevrio looks similar to parts of SARS‑CoV‑2’s genetic code. When the virus tries to multiply, it accidentally inserts Lagevrio into its genes and is unable to copy itself.
Lagevrio is FDA authorized for adults with mild to moderate COVID who are at high risk for developing severe symptoms. But it shouldn’t be prescribed unless other treatments are unavailable or inappropriate for you to take. Children under 18 years old shouldn’t take Lagevrio.
The standard Lagevrio dosage is 4 capsules by mouth every 12 hours for 5 days. There are no dosage adjustments needed for people with liver or kidney problems. Like Paxlovid, you should start taking Lagevrio within the first 5 days of feeling COVID symptoms.
How much do Paxlovid and Lagevrio cost?
The cost of Paxlovid and Lagevrio will depend on whether you have insurance and if you qualify for savings opportunities from the manufacturers.
The average cost of Paxlovid for a 5-day treatment course is $1675.59. Many insurance plans, including Medicare, cover Paxlovid. If you have commercial insurance, a manufacturer copay card can help lower your costs. Paxlovid may cost as little as $0 when using this savings card. If you’re uninsured, you may be eligible for the medication’s patient assistance program, which offers the medication free of cost.
The average cost for 5 days’ worth of Lagevrio is $1174.18. Similar to Paxlovid, many insurance plans, including Medicare, cover Lagevrio. If you have commercial insurance, you may be eligible to pay as little as $10 for the medication using a savings card from the manufacturer. A patient assistance program is also available for Lagevrio.
Are there any Paxlovid or Lagevrio alternatives?
Yes. Veklury (remdesivir) is an alternative to Paxlovid and Lagevrio. It’s FDA approved to treat mild to moderate COVID in adults and children who weigh at least 3.3 lbs and have a high risk for developing severe symptoms. Unlike the COVID pills, Veklury is also approved to treat people who are hospitalized with COVID. But it may be less convenient or accessible for some people.
Veklury is an IV infusion. It must be given at a hospital, an infusion clinic, or a similar healthcare facility. It’s infused into your vein once a day. But an infusion may take between 30 minutes and 2 hours to receive, depending on your dose. If you’re not hospitalized for COVID, you’ll receive Veklury for 3 days. You should start it within 7 days of first feeling symptoms.
Frequently asked questions
Paxlovid most commonly causes a bad taste (“Paxlovid mouth”) and diarrhea. It can also cause headaches and temporary increases in blood pressure. Side effects aren’t common with Lagevrio. But it may cause diarrhea, nausea, and dizziness for some people. More seriously, it may cause bone and cartilage damage in kids, which is why children and teens under 18 shouldn’t take it. Lagevrio can also cause birth defects and shouldn’t be taken if you’re pregnant.
Drug interactions are the biggest reason why some people can’t take Paxlovid. Certain interactions can pose serious risks and lead to severe side effects. People with severe liver disease should also avoid Paxlovid. You shouldn’t take Lagevrio if you’re pregnant since there’s a risk of birth defects. And because there’s a risk of bone and cartilage damage, Lagevrio shouldn’t be prescribed for children under age 18.
You may begin noticing symptom improvement 1 to 2 days after starting Paxlovid. But it may take another few days to feel fully recovered. People taking Lagevrio have reported similar experiences, feeling full symptom relief about 4 days after starting it on average. Regardless of which COVID pill you take, you may still experience lingering symptoms, such as a persistent cough, for several weeks.
The bottom line
Paxlovid (nirmatrelvir / ritonavir) and Lagevrio (molnupiravir) are two oral treatments for mild to moderate COVID-19. They’re available by prescription only. You can get these COVID pills by visiting your primary care provider, an urgent care center, or a Test to Treat location. There’s also a telehealth Test to Treat option.
You may be able to get Paxlovid directly from your pharmacist. To get Paxlovid this way, you’ll need to give your pharmacist copies of recent medical records and lab tests, plus a medication list. This option isn’t available for Lagevrio, which requires a prescription from a licensed practitioner, such as a physician.
Why trust our experts?


References
Administration for Strategic Preparedness and Response. (n.d.). Test to Treat frequently asked questions.
Butler, C. C., et al. (2023). Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet.
Cavazzoni, P. A. (2023). Emergency use authorization 108. U.S. Food and Drug Administration.
Cavazzoni, P. A. (2024). Emergency use authorization 105. U.S. Food and Drug Administration.
Centers for Disease Control and Prevention. (2024). People with certain medical conditions and COVID-19 risk factors.
Gilead Sciences. (2024). Veklury- remdesivir injection [package insert]. DailyMed.
Kozlov, M. (2023). COVID drug Paxlovid was hailed as a game-changer. What happened? Nature.
Lageviro. (n.d.). Learn about our coupon offer for Lagevrio (molnupiravir).
Merck and Co. (2024). Fact sheet for patients and caregivers: Emergency use authorization (EUA) of Lagevrio (molnupiravir) capsules for coronavirus disease 2019 (COVID-19).
Pfizer. (2024). Fact sheet for patients, parents, and caregivers: Emergency use authorization (EUA) of Paxlovid for coronavirus disease 2019 (COVID-19).
Pfizer Laboratories, Division of Pfizer. (2023). Paxlovid- nirmatrelvir and ritonavir [package insert].